THERYA NOTES 2025, Vol. 6: 144-150
First record of Seuratum sp. (Nematoda: Seuratidae) in Chrotopterus auritus (Chiroptera: Phyllostomidae) from Mexico
Primer registro de Seuratum sp. (Nematoda: Seuratidae) en Chrotopterus auritus (Chiroptera: Phyllostomidae) de México
Jesús Alonso Panti-May1*, María Cristina MacSwiney G.2, Jorge Ortega3 and Wilson Isaias Moguel-Chin4
1Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, Universidad Autónoma de Yucatán. Avenida Itzáes 490, C. P. 9700, Mérida. Yucatán, México. Email: alonso.panti@correo.uady.mx (JAP-M).
2Centro de Investigaciones Tropicales, Universidad Veracruzana. José María Morelos y Pavón 44 y 46, Centro, C. P. 91000, Xalapa. Veracruz, México. Email: cmacswiney@uv.mx (MCMG).
3Laboratorio de Bioconservación y Manejo, Posgrado en Ciencias Químico-Biológicas, Departamento de Zoología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional. Prolongación Carpio y Plan de Ayala s/n, Col. Santo Tomás, C.P. 11340, Ciudad de México. Ciudad de México, México. Email: artibeus2@aol.com (JO).
4Doctorado en Manejo de Recursos Naturales Tropicales, Campus de Ciencias Biológicas y Agropecuarias, Universidad Autónoma de Yucatán. Km 15.5 carretera Mérida-Xmatkuil, C. P. 97135, Mérida. Yucatán, México. Email: wilson-im@hotmail.com (WIM-C).
*Corresponding author
Chrotopterus auritus is a rare carnivorous species in the Neotropical region. The helminth fauna of this bat is poorly known, with only 1 published record in Peru. In Mexico, C. auritus is classified as endangered, mainly due to deforestation. The study of helminths in wildlife is relevant, as they can influence behavior, population dynamics, and evolution of their hosts, regulating populations and contributing to the maintenance of biodiversity. As a part of a DNA barcoding survey of bats in Mexico, a specimen of C. auritus was examined for helminths. Only three adult female nematodes were recovered from the intestine of this bat. Nematodes were morphologically studied using light microscopy and scanning electron microscopy. In addition, the 28S ribosomal RNA (28S) gene and the cytochrome c oxidase subunit 1 (COI) gene of 1 specimen were amplified and sequenced. Nucleotide sequences obtained from our specimen were used for phylogenetic analyses. The morphology observed in 2 specimens (e. g. cephalic structures, esophagus, rows of spines, vulva, and tail) agreed with characteristics established for the genus Seuratum. Phylogenetic analyses of the superfamily Seuratoidea grouped our 28S and COI sequences of Seuratum sp. with Paraquimperia sp., a species of the family Quimperiidae. The morphology observed in the female specimens differ from those reported for Seuratum cancellatum, the only species reported from Neotropical bats. This suggests that the specimens found in Yucatan represent a distinct, potentially new species. However, additional specimens, particularly males, are needed to confirm this hypothesis. This study provides the first helminthological record in C. auritus from Mexico, as well as the first 28S and COI sequences for members of the family Seuratidea.
Key words: Carnivorous bat; molecular phylogeny; morphology; nematode; Yucatan.
Chrotopterus auritus es una especie carnívora rara en la región Neotropical. La helmintofauna de este murciélago es poco conocida, con solo 1 registro publicado en Perú. En México, C. auritus está clasificada como amenazada, principalmente debido a la deforestación. El estudio de los helmintos en fauna silvestre es relevante ya que pueden influenciar la conducta, dinámica de población y evolución de sus hospederos, regulando sus poblaciones y contribuyendo al mantenimiento de la biodiversidad. Como parte de un estudio de código de barras de DNA de murciélagos en México, un espécimen de C. auritus fue examinado por helmintos. Solo tres especímenes hembra de nematodos fueron recolectados del intestino de este murciélago. Los nematodos fueron estudiados morfológicamente con microscopía óptica y microscopía electrónica de barrido. Además, el gen 28S de RNA ribosomal (28S) y el gen citocromo oxidasa subunidad 1 (COI) de 1 espécimen fueron amplificados y secuenciados. Las secuencias de nucleótidos obtenidas de nuestro espécimen fueron usadas en análisis filogenéticos. La morfología observada en 2 especímenes (e. g. estructuras cefálicas, esófago, fila de espinas, vulva y ano) concuerdan con las características establecidas para el género Seuratum. Los análisis filogenéticos de la superfamilia Seuratoidea agruparon nuestras secuencias de 28S y COI de Seuratum sp. con Paraquimperia sp., una especie de la familia Quimperiidae. La morfología observada en los especímenes hembra difieren de las características reportadas para Seuratum cancellatum, la única especie reportada en murciélagos neotropicales. Esto sugiere que los especímenes encontrados en Yucatán representan una especie distinta, potencialmente nueva. Sin embargo, se requieren especímenes adicionales, particularmente machos para confirmar esta hipótesis. Este estudio proporciona el primer registro helmintológico en C. auritus en México, así como las primeras secuencias de 28S and COI para miembros de la familia Seuratidea.
Palabras clave: Filogenia molecular; morfología; murciélago carnívoro; nematodo; Yucatán.
© 2025 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
DOI: 10.12933/therya_notes-24-212
ISSN 2954-3614
Chrotopterus auritus (Chiroptera: Phyllostomidae) is one of the largest bats in the Neotropical region (Medellín 2014). Its distribution extends from southeastern Mexico through Central America to South America, reaching northern Argentina (Medellín 1989, 2014). This bat is an opportunistic hunter that primarily feeds on small mammals, birds, and large insects, but it can also consume reptiles, amphibians, fruits, and pollen (Medellín 1988; Vleut et al. 2019). Chrotopterus auritus forms small groups of 1 to 3 individuals that roost in caves, mines, abandoned buildings, hollow termite nests, and hollow trees (Medellín 1989). This bat is considered a rare species threatened in many countries within its range, including Mexico, mainly due to deforestation (Vleut et al. 2019).
Helminths are an important component of ecosystems, as they can shape the behavior, population dynamics and evolution of their hosts, regulating populations and sustaining biodiversity (Smit and Sures 2025). In addition, studying helminths provides insight into the effects of global change, such as climate change and habitat destruction, on disease dynamics (Smit and Sures 2025). Nevertheless, our knowledge of helminths associated with Neotropical bats remains limited, particularly for threatened species.
Knowledge about parasites associated with C. auritus remains limited and varies widely across studies. Ectoparasites, such as flies and ticks, have been reported in several studies on C. auritus from Mexico to Brazil (see Frank et al. 2014; Webb and Loomis 1977). In contrast, there are only 1 published record for this bat species, the trematode Neodiplostomum vaucheri in Peru (Dubois 1983). This contrast may be explained by the fact that ectoparasites can be collected from live animals, whereas helminthological studies typically require the collection of helminths from the organs of recently euthanized hosts, while the helminths are still alive to allow for proper relaxation and fixation (Sepulveda and Kinsella 2013). This poses a particular challenge for conducting helminthological research on threatened host species, such as C. auritus.
In recent years, concerns have been raised about the risk posed by unnecessary collection of organisms, such as bats. This practice can, in some cases, add pressure to already small populations, further threatening and pushing them toward extinction (Russo et al. 2017). To address these concerns, various recommendations have been proposed to promote the comprehensive use of voucher specimens. These include the collection of additional sample types, beyond the primary target samples regardless of the study’s specific goals (Thompson et al. 2021). In recent years, we have formed a research group consisting of chiropterologists and parasitologists to study bat helminths in Mexico. This group seeks to maximize the use of voucher bats used for ecological, genetic, and morphological studies by also gathering additional tissues for helminthological research. In this context, the present study aimed to provide a helminthological record for C. auritus in Mexico.
As a part of a DNA barcoding survey of bats in Mexico, an adult female C. auritus was collected on 9 August 2023 in the Cuxtal Ecological Reserve (2302144.91 N, 232481.23 W), Merida, Yucatan, Mexico. The bat was anesthetized with isoflurane and euthanized with sodium pentobarbital. Stomach, liver and intestines were removed, immersed in 0.9% sodium chloride solution, and examined under a stereomicroscope. Fieldwork was conducted according to the guidelines for the use of wild mammals in research of the American Society of Mammalogists (Sikes and the Animal Care and Use Committee of the American Society of Mammalogists 2016) procedural summaries, and reporting requirements. Included are details on capturing, marking, housing, and humanely killing wild mammals. It is recommended that Institutional Animal Care and Use Committees (IACUCs. The bat specimen was collected under license from the Mexican Ministry of Environment (SEMARNAT) (scientific collection permit SPARN/DGVS/03632/23).
Only three female nematodes were found in the intestine of the bat. Cuts were made from one specimen to extract DNA and examine by scanning electron microscopy (SEM), the other two specimens were cleared and mounted temporally in lactophenol. The morphology of the specimens was studied and drawn with the aid of a light microscope (Leica DM500) with a drawing tube (Leica Microsystems, Wetzlar, Germany). For SEM, fragments of one specimen were dehydrated using a graded ethanol series, critical-point dried with carbon dioxide, sputter-coated with a gold-palladium mixture, and examined at an accelerating voltage of 10 kV with a Hitachi SU1510 (Hitachi, Tokyo, Japan) scanning electron microscope at the Laboratorio de Microscopía y Fotografía de la Biodiversidad, Instituto de Biología, Universidad Nacional Autónoma de México (IBUNAM), Mexico City. One nematode specimen was deposited in the Colección Nacional de Helmintos (CNHE-12235), Universidad Nacional Autónoma de México. Skull and skin of the bat were deposited in the Museo de Zoología of the Universidad Autónoma de Yucatán, Mexico (FMVZ-UADY-1664).
Total genomic DNA of the nematode was extracted using DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). The D1-D3 region of the 28S rRNA gene (28S) and the cytochrome c oxidase subunit 1 (COI) gene were amplified using the polymerase chain reaction (PCR). The 28S fragment was amplified using the primers 391 (Nadler et al. 2003) / 536 (García-Varela and Nadler 2005). The COI fragment was amplified using primers LCO1490/ HC02198 (Folmer et al. 1994). Thermo-cycling profiles followed the protocols described by Hernández-Mena et al. (2017) for 28S and by Folmer et al. (1994) for COI. The PCR primers, along with additional internal primers 503 (Nadler et al. 2003) and 504 (Hernández-Mena et al. 2017) for 28S, were used for Sanger sequencing at Macrogen (Seoul, Korea).
Consensus sequences obtained in this study and other sequences of the superfamily Seuratoidea available in GenBank were used for phylogenetic analyses; trimmed sequences of the domains D2–D3 were used for the 28S gene. The best-fitting nucleotide substitution model was selected for each data set with jModelTest v2, under Akaike information criterion. Phylogenetic affinities for each data set were evaluated by maximum likelihood (ML) analysis with 1,000 bootstrap replicates using RAxML v. 7.0.4. Bootstrap support values were estimated by running 1000 bootstrap resamples. Genetic variation within the 28S and COI data sets was calculated using p-distances with MEGA 11.
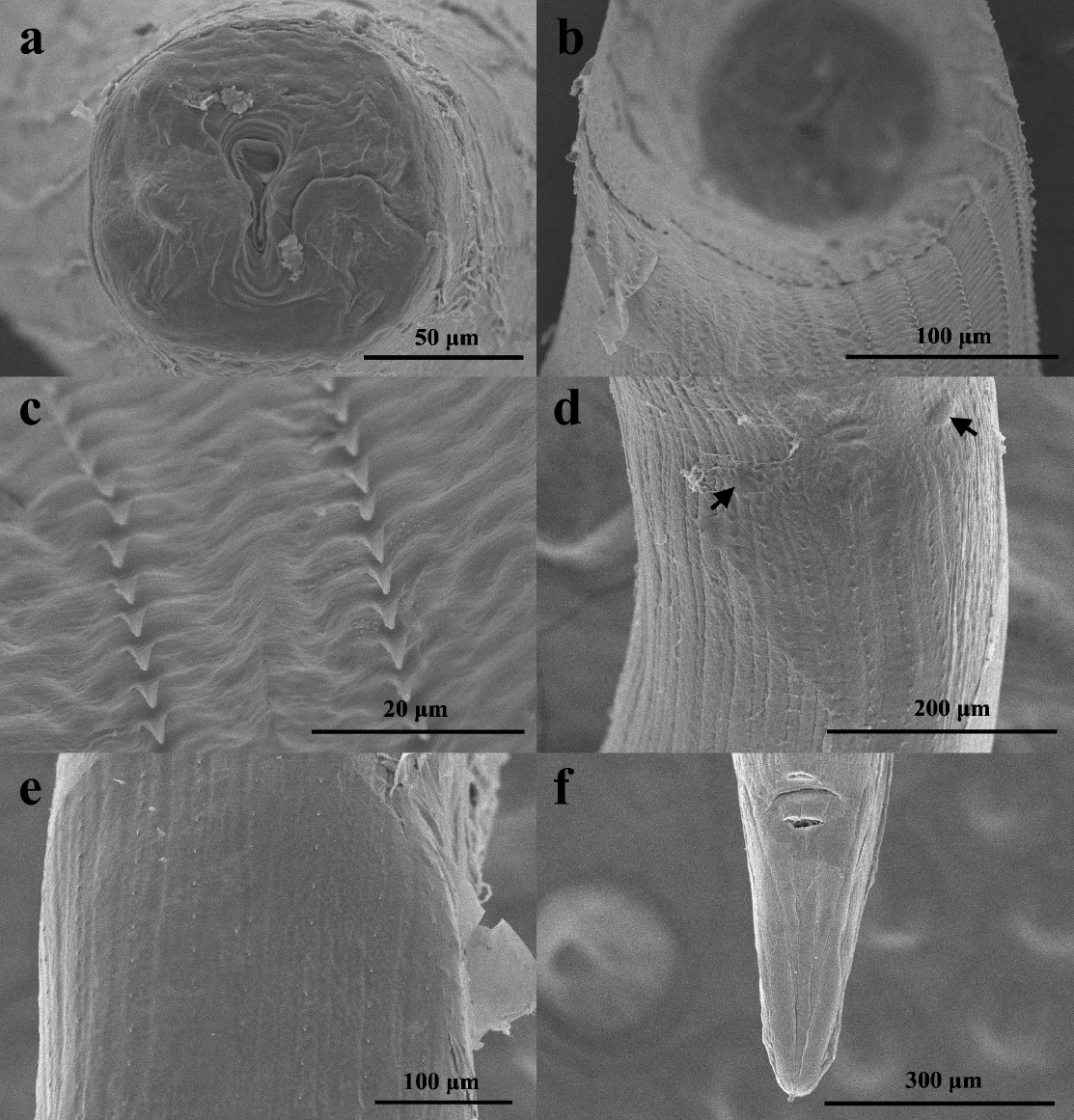
Two gravid specimens (1 adult female and 1 juvenile female) were studied morphologically. Below we present a brief description of the specimens. Triangular oral opening, surrounded by 4 double papillae (Figure 1a). Vestibule and denticles not observed. Esophagus rounded and swollen anteriorly (Figure 2a). Body with 32 rows of cuticular spines (Figure 2b) extending from slightly posterior to nerve ring throughout the mid-body (Figure 2a) and disappearing anterior to anus (Figure. 2c). Spines oriented posteriorly, longer in the anterior region, while in the middle and posterior parts, decreasing in size and the distance between them increases (Figure 1b-e). The main measurements of the adult female are presented in micrometers, followed by those of the juvenile female. Body 26,975 and 11,750 long, and 500 and 230 wide (at vulva level). Esophagus 1,430 and 890 long, and 120 and 68 wide at anterior end. Nerve ring situated 350 and 125 from anterior end. Deirids not observed. Vulva 10,750 and 5660 from anterior end, surrounded by two pairs of papillae located laterodorsally and lateroventrally (Figures 1d, 2d). Anus 570 and 250 from posterior end with short terminal spike 25 and 10 long (Figures 1f, 2c). Eggs with juvenile stage, 38‒40 long by 35‒38 wide, only observed in the adult female.
The 28S sequence generated from Seuratum sp. was 1,164 pb in length (PQ893537). After trimming to the D2-D3 expansion domains, the final alignment was 896 pb long. The 28S sequence of Seuratum sp. was aligned with other 11 sequences of nematodes belonging to the superfamily Seurotoidea. In the resulting phylogenetic tree, Seuratum sp. was positioned as a sister taxon to Paraquimperia sp. (Quimperiidae), isolated from the frog Xenopus laevis, though with low bootstrap support (57) (Figure 3a). The newly generated 28S sequence of Seuratum sp. showed a 19 % genetic difference from that of Paraquimperia sp. Unfortunately, the lack of representativeness of 28S gene sequences has limited the ability to achieve a more comprehensive phylogenetic relationships among the family Seuratidae.
The newly generated COI sequence of Seuratum sp. was 655 pb in length (PQ893890). After trimming to the shortest sequence, the final alignment was 401 bp long. The COI dataset of seuratoid nematodes included 9 sequences. In the phylogenetic tree based on COI, Seuratum sp. was positioned as a sister taxon to Paraquimperia sp. from X. laevis with high support (bootstrap = 100) (Figure 3b). The genetic difference between these taxa was 16.6 %. Unfortunately, the limited availability of COI sequences from members of Seuratidae limited the ability to achieve a resolved phylogeny.
The genus Seuratum includes nine species that parasitize rodents, bats, shrews, and hedgehogs (Noor-un-Nisa et al. 2006). In the Americas, Seuratum cancellatum is the only species previously recorded in bats. This nematode has been reported from Antrozous pallidus, Eptesicus fuscus, Eumops perotis, Myotis californicus, Myotis yumanensis, Parastrellus hesperus (syn. Pipistrellus hesperus), Corynorhinus townsendii (syn. Plecotus townsendii), and Tadarida brasiliensis in Texas (Specian and Ubelaker 1976), Myotis septentrionalis (McAllister et al. 2004), and Myotis leibii (McAllister et al. 2017) in Arkansas, USA, and Natalus mexicanus in Yucatan, Mexico (Chitwood 1938). Unidentified species of Seuratum have been reported from Myotis keaysi in Venezuela (Guerrero 1985), Phyllostomus discolor, Gardnerycteris crenulata, Myotis nigricans, and Lophostoma occidentale in Peru (Minaya Angoma et al. 2020).
The morphological characteristics observed in the female specimens studied align with the genus Seuratum. Compared with S. cancellatum, our specimens have more cuticular spines rows (20 vs. 32) and longer (29‒33 vs. 38‒40) and wider (17‒29 vs. 35‒38) eggs. These differences suggest that the specimens represent a distinct, potentially new species. However, additional specimens, particularly males, are needed to confirm this hypothesis.
The life cycle of Seuratum species parasitizing bats (S. cancellatum, S. mucronatum and S. congolense) remains unknown. Experimental studies have shown that the locust Locusta migratoria (Orthoptera) acts as intermediate host of Seuratum cadarachense, a parasite of the rodent Eliomys quercinus in Europe (Quentin 1970). Similarly, the cockroaches Blatta orientalis and Periplaneta americana (Blattodea) have been identified as intermediate hosts of Seuratum nguyenvanaii, a parasite of the shrew Suncus murinus in Southeast Asia (Le-Van-Hoa 1966). Although C. auritus primarily consumes small vertebrates (Medellín 1988; Bonato et al. 2004), insects (e. g., Coleoptera, Diptera, Lepidoptera) can represent 44 % of its diet, increasing to 75 % during the wet season (Bonato et al. 2004). The presence of the genus Seuratum in C. auritus suggests that some insects in its diet may act as intermediate hosts for these nematodes.
The use of DNA sequences as genetic markers has proven to be useful for species identification, especially when the biological material is limited (Chan et al. 2021). In this study, we provided 28S and COI DNA sequences, complementing the morphological characterization of the female specimens. These barcoding genes have been used to resolve helminth phylogenies, discover cryptic species, and elucidate patterns of gene flow among helminth populations (Poulin et al. 2019). Although morphological analysis was restricted to females, the sequences confirmed the genetic relationship of Seuratum with other nematode species of the superfamily Seuratoidea. These sequences will also serve as a resource for future studies that generate additional data on members of the family Seuratidae.
This study reports the first helminthological record for C. auritus in Mexico and the first published record of the genus Seuratum in this bat species within the Neotropical region. It provides a new helminthological record for C. auritus, an endangered bat species in Mexico, using both morphological and molecular data. Although a specific identification could not be achieved, the information generated will be valuable for future studies on bat helminths.
Acknowledgements
We want to thank to M. I. Chi López for the drawings, A. J. Chan Casanova for her help in the laboratory, and K. G. Martínez-Flores and B. Mendoza-Garfias for the SEM micrographs.
Literatured cited
Bonato, V., K. Gomes Facure, and W. Uieda. 2004. Food habits of bats of subfamily Vampyrinae in Brazil. Journal of Mammalogy 85:708-713.
Chan, A. H. E., et al. 2021. Assessing the suitability of mitochondrial and nuclear DNA genetic markers for molecular systematics and species identification of helminths. Parasites & Vectors 14:233.
Chitwood, B. G. 1938. Some nematodes from the caves of Yucatan. Publications of Carnegie Institute of Washington 491:51-66.
Dubois, G. 1983. Un Néodiplostome péruvien, Neodiplstomum (N.) vaucheri n. sp. (Trematoda: Strigeoidea: Diplostomidae), parasite d’une Chauve-souris. Revue Suisse de Zoologie 90:179-182.
Folmer, O., et al. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294-299.
Frank, R., et al. 2014. Macroparasites of microchiroptera: bat ectoparasites of Central and South America. Pp. 87-130 in Bats (Chiroptera) as vectors of diseases and parasites (Klimpel, S., and H. Mehlorn, eds.). Springer-Verlag, Berlin, Germany.
García-Varela, M., and S. A. Nadler. 2005. Phylogenetic relationships of Palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. Journal of Parasitology 91:1401-1409.
Guerrero, R. 1985. Parasitología. Pp. 35-91 in El estudio de los mamíferos en Venezuela. Evaluación y perspectiva (Aguilera, M., ed.). Fondo Editorial Acta Científica Venezolana, Caracas, Venezuela.
Hernández-Mena, D. I., M. García-varela, and D. Pérez-Ponce de León. 2017. Filling the gaps in the classification of the Digenea Carus, 1863: systematic position of the Proterodiplostomidae Dubois, 1936 within the superfamily Diplostomoidea Poirier, 1886, inferred from nuclear and mitochondrial DNA sequences. Systematic Parasitology 94:833-848.
Le-Van-Hoa. 1966. Cycle évolutif de Seuratum nguyenvanaii Le-Van-Hoa, 1964, parasite de la musaraigne, Suncus murinus (L.) au Viet-Nam. Bulletin de la Société Pathologie Exotique 57:14-128.
Mcallister, C. T., R. S. Seville, and C. R. Bursey. 2017. Helminth (Cestoda, Nematoda) and coccidian (Apicomplexa: Eimeriidae) parasites of the eastern small-footed myotis, Myotis leibii (Chiroptera: Vespertilionidae) from Arkansas, with a description of a new species of Eimeria. Acta Parasitologica 62:139-148.
Mcallister, C., S. J. Upton, and S. R. Bursey. 2004. Parasites (Coccidia, Trematoda, Nematoda) from selected bats of Arkansas. Journal of the Arkansas Academy of Science 58:133-136.
Medellin, R. A. 1988. Prey of Chrotopterus auritus, with notes on feeding behavior. Journal of Mammalogy 69:841-844.
Medellín, R. A. 1989. Chrotopterus auritus. Mammalian Species 343:1-5.
Medellín, R. A. 2014. Big-eared woolly bat. Pp. 692-693 in Mammals of Mexico (Ceballos, G., ed.). Johns Hopkins University Press, Maryland, USA.
Minaya Angoma, D., et al. 2020. Helminth parasites of bats (Chiroptera, Phyllostomidae) in the Department of Junin, Peru and check list of records made in Peru. Revista del Museo Argentino de Ciencias Naturales n.s. 22:57-73.
Nadler, S. A., et al. 2003. Molecular phylogenetics and diagnosis of soil and clinical isolates of Halicephalobus gingivalis (Nematoda: Cephalobina: Panagrolaimoidea), an opportunistic pathogen of horses. International Journal for Parasitology 33:1115-1125.
Noor-Un-Nisa, R. R. Ghazi, and A. Khan. 2006. A new nematode Seuratum bilqeesae from indian gerbil Tatera indica (Hardwicke, 1807). International Journal of Biology and Biotechnology 3:277-280.
Poulin, R., E. Hay, and F. Jorge. 2019. Taxonomic and geographic bias in the genetic study of helminth parasites. International Journal for Parasitology 49:429-435.
Quentin, J. C. 1970. Sur le cycle évolutif de Seuratum cadarachense Desportes, 1947 et ses affinités avec ceux des Nématodes Subulures (Ascaridida) et Rictulaires (Spirurida). Annales de Parasitologie 45:605-628.
Russo, D., et al. 2017. Collection of voucher specimens for bat research: conservation, ethical implications, reduction, and alternatives. Mammal Review 47:237-246.
Sepulveda, M. S., and J. M. Kinsela. 2013. Helminth collection and identification from wildlife. Journal of Visualized Experiments 82:e51000.
Sikes, R. S., and The Animal Care and Use Committee of the American Society of Mammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663-688.
Smit, N. J. and B. Sures. 2025. Introduction to aquatic parasitology: ecological and environmental concepts and implications of marine and freshwater parasites. Pp. 3-8 in Aquatic parasitology: ecological and environmental concepts and implications of marine and freshwater parasites (N. J. Smit and B. Sures, eds). Springer, Cham, Switzerland.
Specian, R. D., and J. E. Ubelaker. 1976. Redescription of a nematode, Seuratum cancellatum Chitwood, 1938, from bats in Texas. Proceedings of the Helminthological Society of Washington 43:59-65.
Thompson, C. W. et al. 2021. Preserve a voucher specimen! the critical need for integrating natural history collections in infectious disease studies. mBio 12:e02698-20.
Vleut, I., G. G. Carter, and R. A. Medellín. 2019. Movement ecology of the carnivorous woolly false vampire bat (Chrotopterus auritus) in southern Mexico. PLoS ONE 14:e0220504.
Webb, J. P. J., and R. B. Loomis. 1977. Ectoparasites. Pp. 57-119 in Biology of bats of the New World family Phyllostomatidae. Part II (Baker, J. R., J. K. Jones, and D. C. Carter, eds.). Texas Tech University, Texas, USA.
Associate editor: Daily Martínez Borrego
Submitted: April 7, 2025; Reviewed: September 4, 2025.
Accepted: September 9, 2025; Published on line: October 09,
2025
Figure 1. SEM micrographs of females of Seuratum sp. a) Head, apical view. b) Anterior part, apical view. c) Spines of the anterior part, ventral view. d) Middle section of the body showing the vulva and two latero-ventral papillae (arrows), ventral view. e) Posterior section of the body, ventral view. f) Posterior part, ventral view.

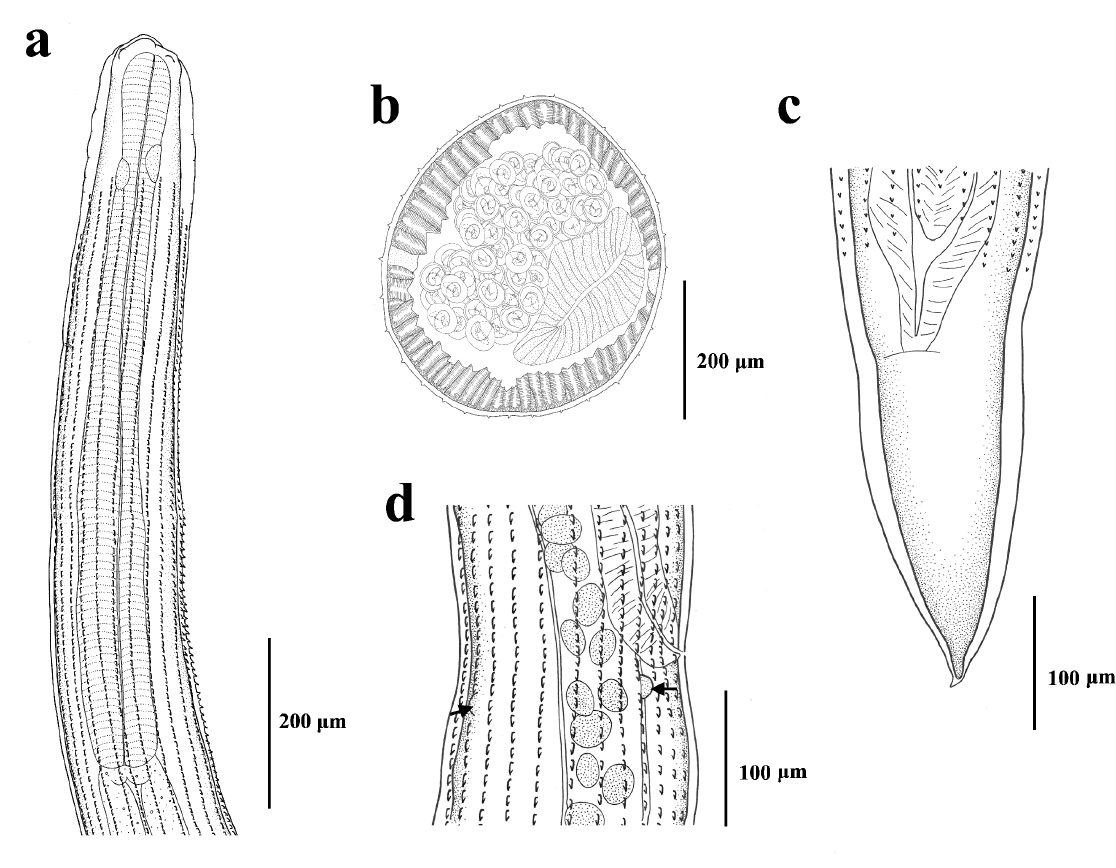
Figure 2. Drawings of females of Seuratum sp. a) Anterior part, lateral view. b) Transverse section, at mid-body. c) Posterior part, ventral view. d) Vulvar region, showing two papillae (arrows), right lateral view.

Figure 3. Maximum likelihood (ML) phylogenetic trees of Seuratoidea. a) Inferred with 28S sequence data using the TIM3 + G model (In likelihood -6879.440063). b) Inferred with COI sequence data using the GTR + G model (In likelihood -2249.908230). GenBank accession numbers precede species name, followed by host name. Bootstrap support values for ML are provided at the nodes. The new sequence of the present study is in bold.