Plant and animal interactions play an essential role in the ecology and evolution of species and the preservation and restoration of balanced ecosystems (Kunz et al. 2011; Sil et al. 2023). Mammals such as bats (Order Chiroptera) are fundamental in agricultural ecosystems, where they fulfill essential ecological roles such as pest control, plant pollination, and seed dispersal (Laurindo et al. 2020; Ocampo-Ariza et al. 2022). Fruit bats in particular consume fruits completely or partially, allowing the seeds to pass through their digestive system and be dispersed through their feces (Voigt et al. 2009). For fruits with seeds too large for bats to consume, dispersal occurs as bats move between the food source and place of consumption, often roosts (Melo et al. 2009). In both ways, bats contribute to the regeneration and propagation of plants with morphologically distinct fruits (Melo et al. 2009).
Several studies have documented the ability of species in the genus Artibeus to transport fruits weighing comparable to, or even greater than, their own body mass. Individuals of A. jamaicensis and A. lituratus have been reported transporting or consuming guava (Psidium guajava) fruits weighing up to 50 g, and even avocado (Persea americana) fruits weighing an estimated 71.7 g (Gardner 1977; Hernández-Mijangos and Medellín 2009; Duque-Márquez and Muñoz-Romo 2015). The transportation of whole fruits in the mouth, without being completely ingested, is known as stomatochory (McConkey et al. 2024). This seed dispersal mechanism moves fruits or seeds from the source plant to a consumption or resting site, promoting plant regeneration at a distance (McConkey et al. 2024).
Artibeus fraterculus is a species of bat in the Phyllostomidae family (Subfamily Stenodermatinae) endemic to the Tumbes region of Peru and Ecuador (Marques-Aguiar 2008; Salas et al. 2018). In Peru, it has been reported along the Pacific coast from Tumbes to Ica, in the Andes, and in arid ecosystems in the Amazon basin in the Cajamarca and Amazonas regions (Ortiz de la Puente 1951; Tuttle 1970; Koopman 1978; Pacheco et al. 2007; Salas et al. 2018). In Ecuador, it has been reported in the central and southern coast from 0 to 1,600 m, with one report in an area of humid montane scrubland (Salas et al. 2018).
In situ and field observations remain important for understanding the biology and natural history of bats (Hernández-Mijangos and Medellín 2009; Carrasco-Escudero and Hughes 2025). For example, information on folivory in A. fraterculus has been reported primarily from observations in roosts or mist nets at the time of capture (Ruiz-Ramoni et al. 2011; Duque-Márquez et al. 2019; Arias and Aguirre 2022).
There are several studies on the diet of A. fraterculus (Novoa et al. 2011; Pinto et al. 2013); however, very little or nothing is known about its feeding behavior despite the implications for seed dispersal and intraspecific interactions (Salas et al. 2018). In Artibeus lituratus, males defend their roosts while conducting short foraging sessions to consume food in the roost itself. In contrast, females travel to more remote foraging locations and are not observed feeding at or near roosts (Muñoz-Romo and Herrera 2010). Furthermore, there are no dietary studies of fruit bats in the Marañón Valley, a region with a climate and botanical diversity distinct from those of the coast (Marcelo-Peña et al. 2016).
The main objective of this study is to report the maximum recorded fruit weight carried by the fraternal fruit bat A. fraterculus. In addition, we report for the first time the location of a foraging roost and the fruits consumed on the eastern slope of the Andes in two locations in the department of Cajamarca.
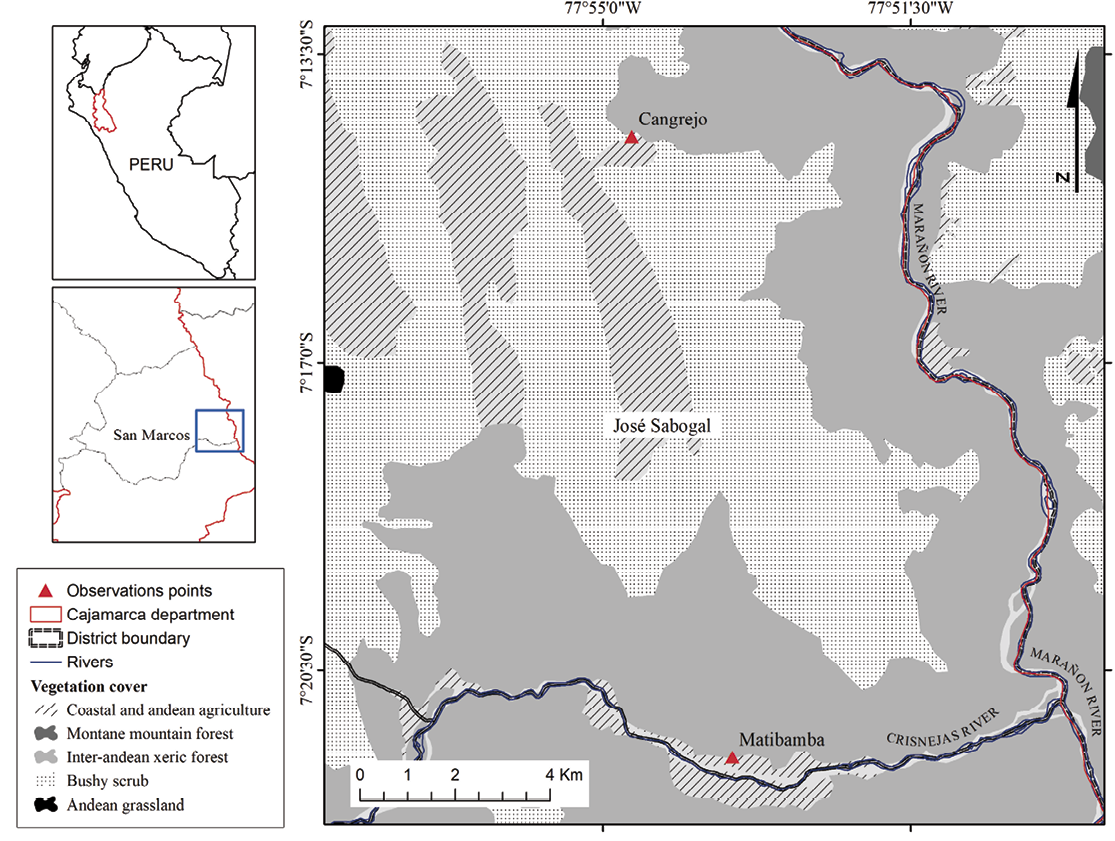
Observations were made at two locations in the José Sabogal district, San Marcos province, Cajamarca region (Figure 1). The first, Matibamba (07° 21’ 29’’ S, 77° 53’ 32’’ W), is a populated center located in the extreme southeast of the district, with an average altitude of 1,160 m and an annual rainfall of approximately 670 mm (Gobierno Regional de Cajamarca [GRC] 2020; Fick and Hijmans 2017). The second, Cangrejo (07° 14’ 26’’ S, 77° 54’ 40’’ W), is a populated center located 13.2 km north of Matibamba with an average altitude of 2,000 m and an annual rainfall of approximately 750 mm (Fick and Hijmans 2017).
Both locations are within the Marañón Seasonally Dry Forest ecosystem, characterized by a sub-humid and semi-warm climate, seasonally deciduous vegetation, and columnar cacti (Ministry of the Environment [MINAM] 2018; GRC 2020; Linares-Palomino et al. 2022). The average canopy height varies between both locations due to climate as well as current and historical land use. Canopy height averaged 13 meters in height in Matibamba and 27 meters in Cangrejo (data extracted from Potapov et al. 2022). Agricultural production in these areas includes coca, rice, cassava, corn, and beans, as well as fruit trees such as mango, lemon, banana, plum, orange, and cocoa (GRC 2020).
During bat mist-net surveys at both locations, several individuals of the fraternal fruit bat (A. fraterculus) were observed perched on tree branches while feeding. These observations occurred coincidentally during the capture work and were documented using a handheld flashlight and camera. A. fraterculus is easily distinguished from other Artibeus species due to their faint facial lines, an overall grayish coloration with a paler ventral region, and a smaller size than other species in the Artibeus genus (Tirira 2017; Salas et al. 2018). At these locations, A. lituratus and A. planirostris are the most similar species to A. fraterculus. A. lituratus is distiguishable by their larger size, more pronounced facial lines, and an overall browner coloration (Tirira 2017); while A. planirostris is more robust, and its facial lines may be less evident (Hollis 2005).
Additionally, we weighted fruits taken from the areas used as feeding grounds. For partially consumed Psidium guajava fruit, the missing portion was visually estimated by comparing its shape and volume with the expected morphology of a whole fruit of the same species in order to estimate the original weight (Duque-Márquez and Muñoz-Romo 2015). Fruit was weighed using a Pesola Lightline dynamometer with a maximum capacity of 100 g to ensure accurate measurements.
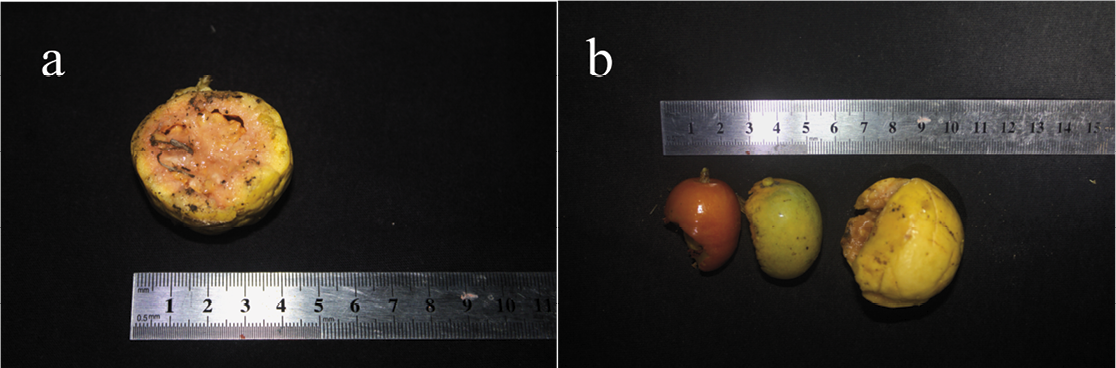
On February 1, 2024, at 10:30 p.m., in the Cangrejo locality, individuals of A. fraterculus were observed perching and feeding on Eriobotrya japonica (loquat, Figure 2a) fruits. The fruits consumed had an average weight of 7.3 g ± 1.58 g. Individuals were perched 4 to 10 m from the nearest E. japonica tree.
In Matibamba, on February 8, 2024, at midnight, one A. fraterculus individual was observed perching on the branch of a tamarind tree (Tamarindus indica; Figure 2b), feeding on a guava (Psidium guajava) fruit. We observed the individual drop the fruit after feeding on it. The fruit displayed bite marks, and its weight was recorded at 31 g. It is estimated that approximately one-third of the fruit had been consumed (Figure 3a). Based on this estimate, the original fruit weight was calculated at 45 g. Weights of A. fraterculus range from 30 to 55 g (Salas et al. 2018), with the median weight of 42.5 g used as a reference here. Thus, the weight of the consumed fruit would represent approximately 105.88 % of the estimated average body weight for the species.
Additionally, at the Matibamba location, remains of other fruits showing signs of having been consumed by bats were found, including Spondias purpurea (plum, 9 g), Mangifera indica (mango, 14 g), and P. guajava (guava, 26 g; Figure 3b). These findings indicate a diverse frugivorous diet for A. fraterculus at the Matibamba locality and may indicate a partial dependence on agricultural species.
Other bat species were captured at each location on the same night. Individuals of Sturnira giannae, A. planirostris, Promops davisoni, Glossophaga valens, and Carollia perspicillata were recorded at Matibamba. At Cangrejo, G. valens, C. perspicillata, and A. planirostris were captured. Individuals of Carollia sp. and A. fraterculus were observed sharing a branch to feed.
Several species of the genus Artibeus have been documented to be able to carry fruit that, in some cases, is comparable to their own body mass. For example, Gardner (1977) reported an individual of A. jamaicensis in Colombia carrying a guava fruit weighing 50 g. Similarly, Hernández-Mijangos and Medellín (2009) observed an individual of A. lituratus feeding on a guava fruit weighing approximately 50 g, while Duque-Márquez and Muñoz-Romo (2015) reported an individual of A. lituratus consuming a Persea americana (avocado) fruit with an estimated weight of 71.7 g. In addition, diameter measurements have been recorded on transported fruits including by Tuttle (1970) in Peru, where a female A. jamaicensis was carrying a fig fruit approximately 30 mm in diameter. These studies highlight that transported fruits can represent a weight equivalent to the bat’s body mass (Duque-Márquez and Muñoz-Romo 2015). This behavior could reduce the frequency with which bats need to forage, optimizing their energy expenditure (Fleming 1982).
In this context, our study reveals that A. fraterculus, one of the smallest species in the large Artibeus group (Salas et al. 2018), could be capable of carrying fruits equivalent to 1.05 times its body mass, demonstrating its remarkable carrying capacity relative to its size. Furthermore, this species plays an important role in the dispersal of large seeds such as S. purpurea and M. indica through stomatochory (McConkey et al. 2024; Sánchez-Calderón et al. 2025). The ability of A. fraterculus to transport fruits to feeding (night) and day roosts, and to expel undigested seeds in different locations, promotes the connectivity of fragmented habitats. This is especially valuable in tropical ecosystems, where the natural regeneration of native fruit trees may depend on species such as A. fraterculus among others.
The role of fruit bats as dispersers of large seeds has significant implications for ecosystem regeneration (Fleming and Heithaus 1981; Heithaus 1982). Many tree species that produce large-seeded fruits, such as S. purpurea and M. indica, might rely on fruit bats for dispersal. For native species such as P. guajava, this behavior may be crucial for their reestablishment in disturbed landscapes, where plants face greater difficulties in dispersal by birds, rodents, or other mammals due to the loss of dispersing fauna and the tendency of these groups to avoid open areas (Gardner 1977; Kunz et al. 2011; Brändel et al. 2020). For non-native species such as M. indica, this behavior may increase the risk of their naturalization, posing a threat to native species in areas with high endemism (Jiang et al. 2022). However, since these non-native plants can provide alternative food sources for wildlife during the recovery period of native forest diversity, the risk of naturalization must be assessed on a case-by-case basis, considering the area’s management objectives. These complex interactions between agriculture and native biodiversity highlight the importance of promoting collaborative discussions and developing integrated management plans for communities located within and near protected areas. These observations are consistent with the documented tendency of this species to feed on fruits available in each location, even if they are non-native or agricultural species (Salas et al. 2018).
Dietary studies of A. fraterculus have been conducted primarily through the analysis of fecal samples (Novoa et al. 2011) or fruits and seeds collected from shelters (Pinto et al. 2013). In this study, we collected large-seeded fruits from feeding (night) roosts, which may be too heavy to be transported to their shelters and, due to their seed size, may not be entirely ingestible. These fruits would not be reported through seed analysis in fecal samples.
Based on the observations and descriptions of A. lituratus by Muñoz-Romo and Herrera (2010), A. fraterculus would be expected to display sex-specific foraging behavior, with males making short flights to return to their shelter to feed and defend it; while females forage farther away. If true, we would expect feeding (night) roosts to be utilized disproportionately by females while males are feeding in and near permanent day roosts. In the town of Matibamba, 1 km to the northwest, we found a cave inhabited by A. fraterculus. Fruit and seed remains of S. purpurea and M. indica, as well as leaf debris, were observed here, supporting the observations detailed here. However, additional studies to delve deeper into these behaviors are needed to fill the current gaps in knowledge about the natural history of A. fraterculus.
This information contributes to our understanding of the trophic ecology of fruit bats and provides evidence of how fruits with large seeds, which cannot be digested by bats, such as loquat, mango, and plum, can be dispersed by this taxa through stomatochory (McConkey et al. 2024; Novoa et al. 2011; Pinto et al. 2013). This could also occur with fruits of similar or larger size in environments not disturbed by human presence. The coexistence between human crops and native flora could benefit from the presence of A. fraterculus, reinforcing its role as a key seed disperser in tropical ecosystems including those subject to extensive fragmention and agricultural development.
Acknowledgments
We thank the Regional Government of Cajamarca (GRC), especially the Office of the Sub-Management of Natural Resources and Protected Natural Areas, for the logistical support provided to access the study area. We are also grateful to D. Rosario for logistical assistance and accompanying us to the survey localities. Special thanks to M. Flores and F. Jiménez for the facilities provided in the localities of Matibamba and Cangrejo, respectively. Finally, we thank M. Vera Concha, S. Diane Suarez, and R. Elejalde for their assistance during fieldwork.
Literature cited
Arias, E., y M. Aguirre. 2022. Primer registro de folívora en Artibeus fraterculus y Artibeus planirostris en áreas de bosques secos del noreste del Perú. Folia Amazónica 31:135-147.
Brändel, S. D., et al. 2020. Consequences of fragmentation for Neotropical bats: The importance of the matrix. Biological Conservation 252:1-8.
Carrasco-Escudero, L., y M. Hughes. 2025. Notable comportamiento social en el murciélago nectarívoro occidental Lonchophylla hesperia G.M. Allen, 1908 (Chiroptera: Phyllostomidae) en el Coto de Caza El Angolo, Perú. Notas sobre Mamíferos Sudamericanos 7:e25.1114.
Duque-Márquez, A., et al. 2019. Bat folivory in numbers: how many, how much, and how long? Acta Chiropterologica 21:183-191.
Duque-Márquez, A., y M. Muñoz-Romo. 2015. Registro máximo de carga de fruto en murciélagos frugívoros: Artibeus lituratus (CHIROPTERA: PHYLLOSTOMIDAE). Revista Mexicana de Mastozoología Nueva época 5:96-100.
Fleming, T. H. 1982. Foraging strategies of plant visiting bats. Pp. 287-325 in Ecology of bats (Kunz, T.H. ed.). Plenum Press, New York, EE.UU.
Fleming, T. H., y E. R. Heithaus. 1981. Frugivorous bats, seed shadows and the structure of tropical forests. Reproductive Botany 13:45-53.
Fick, S. E., y R. J. Hijmans. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37:4302-4315.
Gardner, A. L. 1977. Feeding habits. Pp. 293-350 in Biology of the bats of the New World family Phyllostomidae. Part II (Baker, R. J., J. K. Jones, y D. C. Carter, eds.). Special Publications The Museum Texas Tech University Press. Lubbock, Texas, EE.UU.
Gobierno Regional de Cajamarca – GRC. 2020. Expediente técnico del Área de Conservación Regional Bosques secos del Marañón. Cajamarca. 24 de noviembre de 2024. Disponible en carrasco.luiggialessandro@gmail.com.
Heithaus, E. R. 1982. Chapter 9. Coevolution between bats and plants. Pp. 327-367 in Ecology of bats (Kunz T. H. ed.). Springer, Boston, MA.
Hernández-Mijangos, L. A., y R. A. Medellín. 2009. Observaciones sobre el consumo de fruto de Psidium guajava por Artibeus lituratus. Revista Mexicana de Mastozoología 13: 105-108.
Hollis, L. 2005. Artibeus planirostris. Mammalian Species 775:1-6.
Jiang, Y., A. A. Polat, y S. Lin. 2022. Origin, History and Production. Pp. 1-20 in Loquat: Botany, Production and Uses (Polat, A. A., y S. Mitra. eds.). GB: CABI. London, United Kingdom.
Koopman, K. F. 1978. Zoogeography of peruvian bats, with special emphasis on the role of the Andes. American Museum Novitates 2651:1-40.
Kunz, T. H., et al. 2011. Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223:1–38.
Laurindo, R. S., et al. 2020. Feeding habits define habitat use by bats in an agricultural landscape of the Atlantic Forest. Revista Mexicana de Biodiversidad 91:e913223.
Linares-Palomino, R., et al. 2022. Los bosques esta-cionalmente secos del Perú: un re-análisis de sus patrones de diversidad y relaciones florísticas. Revista peruana de biología 29: e21613.
Marcelo-Peña, J. L., et al. 2016. Identifying conservation priority areas in the Marañón valley (Peru) based on floristic inventories. Edinburgh Journal of Botany 73:95-123.
Marques-Aguiar, S. A. 2008. Genus Artibeus Leach, 1821. Pp. 301–321 in Mammals of South America: volume 1. Marsupials, xenarthrans, shrews, and bats (A. L. Gardner, ed.). University of Chicago Press, Chicago, Illinois, EE.UU.
McConkey, K. R., H. S. Sushma, y A. Sengupta. 2024. Seed dispersal by frugivores without seed swallowing: Evaluating the contributions of stomatochoric seed dispersers. Functional Ecology 38:480–499.
Melo, F. P., et al. 2009. Small tent‐roosting bats promote dispersal of large‐seeded plants in a Neotropical Forest. Biotropica 41:737-743.
Ministerio del ambiente - MINAM. 2018. Mapa Nacional de Ecosistemas del Perú: Memoria descriptiva. Lima, Perú. 29 de diciembre de 2024.
Muñoz-Romo, M., y E. A. Herrera. 2010. Observaciones sobre la alimentación del murciélago frugívoro mayor Artibeus lituratus (Chiroptera: Phyllostomidae). Revista Mexicana de Mastozoología 14:51-58.
Novoa, S., R. Cadenillas, y V. Pacheco. 2011. Dispersión de semillas por murciélagos frugívoros en bosques del Parque Nacional Cerros de Amotape, Tumbes, Perú. Mastozoología Neotropical 18:81-93.
Ocampo-Ariza, C., et al. 2022. Trait-dependent responses of birds and bats to season and dry forest distance in tropical agroforestry. Agriculture, Ecosystems & Environment 325:107751.
Ortiz de la Puente, D. J. 1951. Estudio monográfico de los quirópteros de Lima y sus alrededores. Publicaciones del Museo de Historia Natural “Javier Prado”. Universidad Nacional Mayor de San Marcos, Serie Zoología 7:1-48.
Pacheco, V., et al. 2007. Noteworthy bat records from the Pacific tropical rainforest region and adjacent dry forest in northwestern Peru. Acta Chiropterologica 9:409–422.
Pinto, M., et al. 2013. Distribution, abundance and roosts of the fruit bat Artibeus fraterculus (Chiroptera: Phyllostomidae). Acta Chiropterologica 15:85-94.
Potapov, P., et al. 2022. The global 2000-2020 land cover and land use change dataset derived from the Landsat archive: first results. Frontiers in Remote Sensing 3:856903.
Ruiz-Ramoni, D., et al. 2011. Folivory in the giant fruit-eating bat Artibeus amplus (Phyllostomidae): a non-seasonal phenomenon. Acta Chiropterologica 13:195-199.
Salas, J. A., C. R. Loaiza, y V. Pacheco. 2018. Artibeus fraterculus (Chiroptera: Phyllostomidae). Mammalian Species 962:67-73.
Sánchez-Calderón, R., et al. 2025. Large-seeded plants dispersed by tent-roosting bats along an altitudinal gradient in Costa Rica. Oecologia 207:95
Sil, S., et al. 2023. Effects of habitat and fruit scent on the interactions between short-tailed fruit bats and Piper plants. Integrative Organismal Biology 6:obae028.
Tirira, D. 2017. Guía de campo de los mamíferos del Ecuador. Ediciones Murciélago Blanco. Publicación especial sobre los mamíferos del Ecuador 6, Quito, Ecuador.
Tuttle, M. D. 1970. Distribution and zoogeography of Peruvian bats, with comments on natural history. The University of Kansas Science Bulletin 49:45-86.
Voigt, C. C., et al. 2009. Dietary analysis of plant-visiting bats. Pp. 593-609 in Ecological and behavioral methods for the study of bats (T. H. Kunz, y S. Parsons. eds.). The Johns Hopkins University Press, Baltimore, Maryland.
Associate editor: Luis Darcy Verde Arregoitia
Submitted: January 24, 2025; Reviewed: May 31, 2025.
Accepted: June 16, 2025; Published online: August 28, 2025.