The kinkajou (Potos flavus) is a medium-sized arboreal mammal that primarily feeds on fruits, flowers, nectar, leaves, and occasionally arthropods. While interactions between P. flavus and plants are well-documented with the species known to consume fruits and other parts of ١١٩ plant species across ٥٠ families, reports of frugivory involving kinkajous and Araceae plants are scarce. To date, only a single instance of frugivory on Philodendron crassispathum has been recorded. During a field trip in March 2022 to Los Tuxtlas, Veracruz, México, we documented the first observed consumption of Monstera egregia by kinkajous using a video camera. The kinkajou was observed perched on a leaf stem of the same M. egregia, approximately 6 m above the ground. The individual gripped the stem with its feet and prehensile tail while using its hands to manipulate the infructescence. The animal was seen biting into the infructescence and picking individual fruits, which it brought to its mouth. Although Araceae plants have evolved mechanisms to deter herbivory, the consumption of M. egregia by kinkajous appears to be a deliberate behavior rather than a random occurrence. Interactions between Araceae species and kinkajous, as well as with other frugivorous mammals, may represent an important ecological relationship deserving of further investigation.
Key words: Diet; frugivory; Los Tuxtlas Biosphere Reserve; plant-animal interaction; Procyonidae.
La martucha (Potos flavus) es un mamífero arbóreo de tamaño mediano que se alimenta principalmente de frutos, flores, néctar, hojas y, ocasionalmente, artrópodos. Aunque las interacciones entre P. flavus y las plantas están bien documentadas con registros de consumo de frutos y otras partes de ١١٩ especies de plantas pertenecientes a ٥٠ familias, los reportes de frugivoría que involucran martuchas y plantas de la familia Araceae son escasos. Hasta la fecha, solo se ha registrado un caso de frugivoría en Philodendron crassispathum. Durante un viaje de campo en marzo de 2022 a Los Tuxtlas, Veracruz, México, documentamos por primera vez el consumo de Monstera egregia por martuchas utilizando una cámara de video. Observamos a una martucha posada sobre el pecíolo de una hoja de una planta de M. egregia, aproximadamente a 6 m de altura. El individuo se sujetaba al pecíolo con los pies y la cola prensil mientras utilizaba sus manos para manipular la infrutescencia. Se le observó mordiendo la infrutescencia y recogiendo frutos individuales, que luego llevaba a su boca. Aunque las plantas de la familia Araceae han desarrollado mecanismos para disuadir la herbivoría, el consumo de M. egregia por martuchas parece ser un comportamiento deliberado más que un evento aleatorio. Las interacciones entre especies de Araceae y martuchas, así como con otros mamíferos frugívoros, podrían representar una relación ecológica importante que merece mayor investigación.
Palabras clave: Dieta; frugivoría; interacción planta-animal; Procyonidae; Reserva de la Biosfera de Los Tuxtlas.
© 2025 Asociación Mexicana de Mastozoología, www.mastozoologiamexicana.org
The kinkajou (Procyonidae: Potos flavus), also known as “martucha”, “micoleón”, or “cuchumbí” is a medium-sized arboreal mammal inhabiting most tropical forests of Central and South America, extending from central Brazil and Bolivia, eastern Perú, the Guiana Shield, and Colombia, northward through Central America and into México, in the tropical forests along the slopes of the Pacific and the Gulf of México (Ford and Hoffmann 1988). This species feeds primarily on fruits (90 % of its diet), with a preference for berries and drupes (Julien-Laferrière 1999; Kays 1999), supplemented by flowers (Estrada and Coates-Estrada 1985; Julien-Laferrière 1993; Kays 1999), nectar (Julien-Laferrière 2001), buds (Villa-Ramírez 1944; Kays 1999), and occasionally arthropods (Bisbal 1986; Red ford et al. 1989).
While kinkajous primarily consume both mature and immature fruits (Julien-Laferrière 1993; Kays 1999), they typically do not digest the seeds, which pass through their short intestines intact and are dispersed away from the parent tree (Julien-Laferrière 2001; Lambert et al. 2014), making them effective seed dispersers in tropical forests (Julien-Laferrière 2001; Galvis et al. 2024).
The ecological relationships between kinkajous and plants, particularly regarding their diet, have been extensively documented (e. g., Kays 1999; Julien-Laferrière 2001; Galvis et al. 2024). It is known that P. flavus feeds on fruits, flowers, or nectar from at least 119 plant species across 50 families (Appendix 1). It also consumes insects and small vertebrates (Appendix 2). However, due to their nocturnal behavior (Ford and Hoffmann 1988) and tendency to avoid passive monitoring (Schipper 2007), the full range of plant species consumed may be underestimated.
The genus Monstera Adans. includes nearly 70 recog-nized hemi-epiphytes plant species distributed across the Neotropics (Govaerts et al. 2021; Govaerts 2024) especially diverse in Central America (Croat et al. 2024). It is conspicuously recognized for their leaf lamina exhibiting fenestrations (i.e., perforations) in many species (but not restricted to this genus; Madison 1977). Like other members of the Araceae family, Monstera species employ a defense mechanism using insoluble calcium oxalate crystals found mainly in bundles in different floral parts, mostly in the spathe and in the inflorescence (Jdeed et al. 2024), deterring herbivores (Madison 1977, 1979; Coté and Gibernau 2012). Frugivory on Monstera by birds has been documented (Madison 1977; Cedeño-Fonseca et al. 2020), and Geoffroy’s Tamarin monkeys (Saguinus geoffroyi) have been observed feeding on Monstera adansonii in Panamá (Cedeño-Fonseca et al. 2020). However, consumption of Monstera species by other mammals remains largely undocumented.
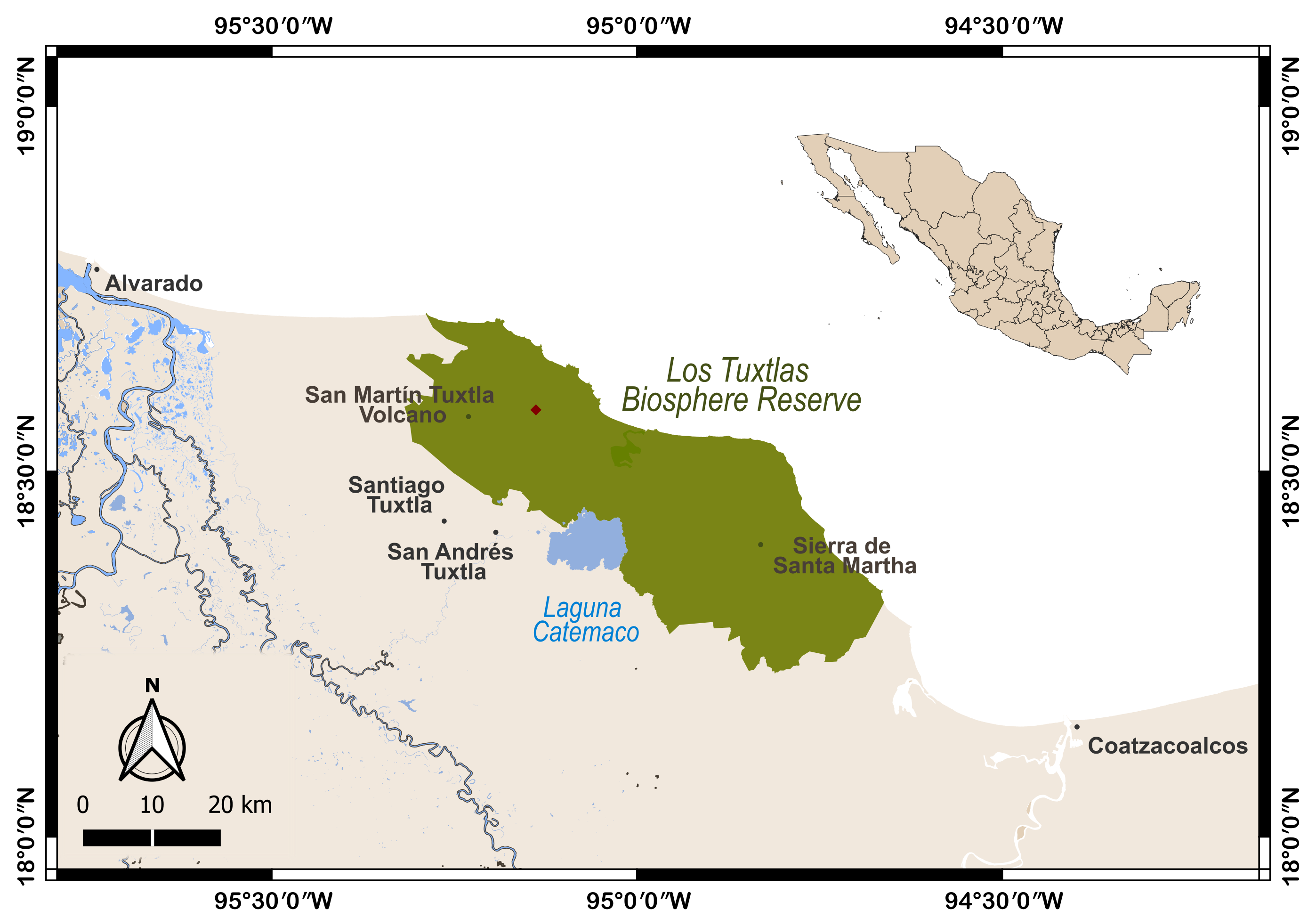
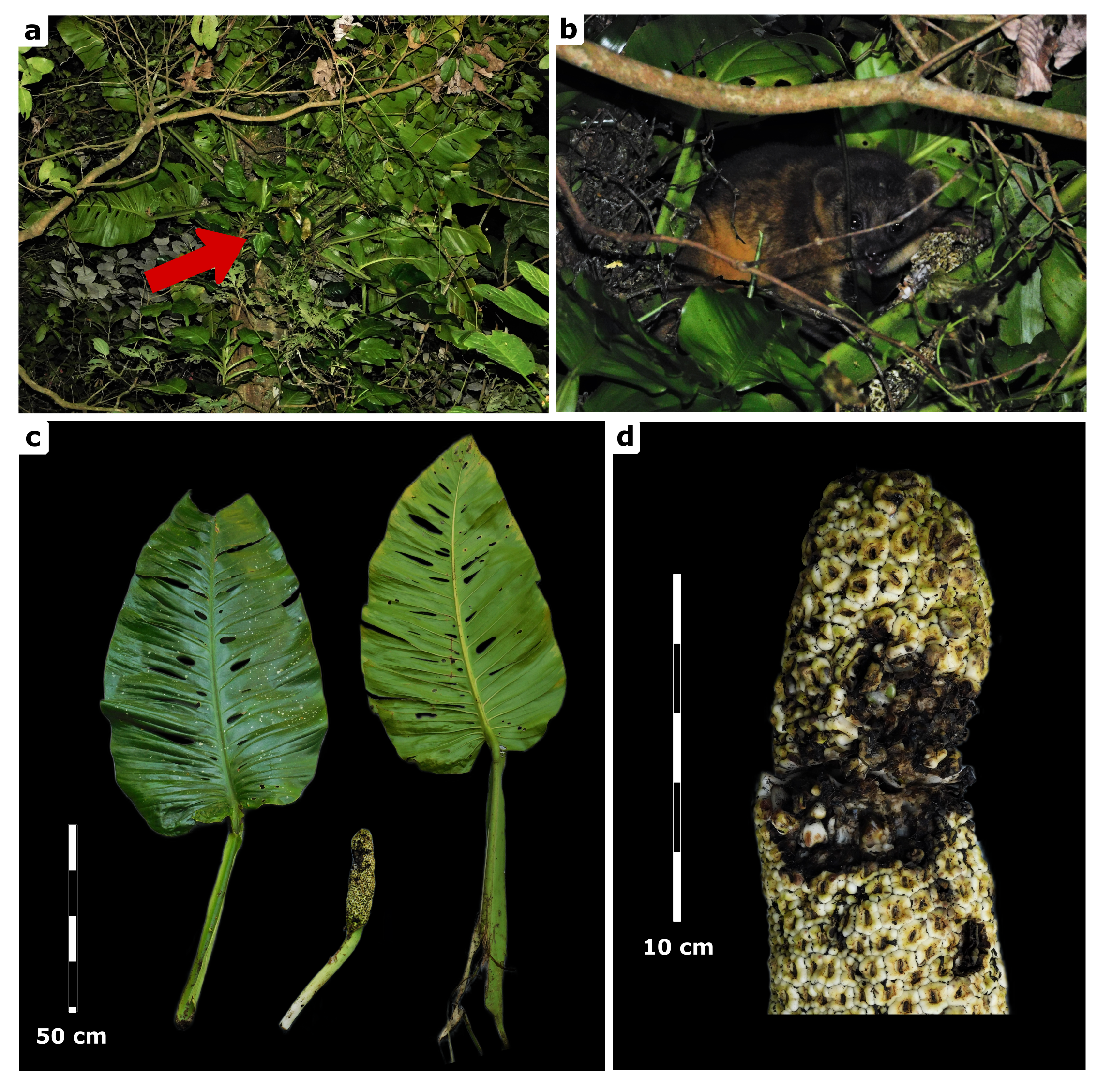
In this study, we report the first documented consumption of ripe fruits from Monstera egregia Schott by P. flavus. The plant is an endemic species for México (Acebey and Krömer 2008), formerly included in Monstera lechleriana (Madison 1977), and is characterized by the leaves aggregated at the apex of the stem. The infructescence of this species ranges from 20 to 28 cm in length and 4.5 to 5.5 cm in diameter. The berries are typically 16-20 mm long and 7-9 mm wide. They are usually one-seeded, oblong in shape, and measure 10-13 mm in length, 4-6 mm in width, and 3.4 mm in thickness (Madison 1977). This event occurred during a survey of the bat fauna in the Los Tuxtlas region (March 9, 2022), located in Southeastern México. The site (18° 32′ 3.55″ N, 95° 8′ 18.54″ W; 1,034 m) is located on the San Martín Tuxtla volcano within the Los Tuxtlas Biosphere Reserve, Veracruz, México (Figure 1). The area has a temperate and humid climate, with tropical montane cloud forest vegetation surrounded by a matrix of pastureland (Guevara et al. 2004).
The kinkajou, whose sex and age could not be deter-mined, was observed twice near a mist net during a bat survey. The first sighting occurred over trees located between a paddock’s wire fence and a rural road. No feeding activity was noted at that time. Although the kin-kajou noticed our presence, it did not attempt to escape. Minutes later, around 21:05 hr, the kinkajou was seen again a few meters away from the initial location, but on the opposite side of the rural road. This time, it was observed feeding on the ripe fruits of an adult M. egregia growing on an unidentified tree, approximately 6 m above the ground (Figure 2). Photographs and video were taken with a Nikon Coolpix B500 camera (Nikon, Inc., Japan).
The kinkajou was positioned on a leaf stem of the same M. egregia, gripping it with its feet and prehensile tail. It frequently used its hands to hold the infructescence while biting into it and to pick individual fruits and bring them to its mouth (see Appendix 3). Although the kinkajou occasionally used its hands to access individual berries, most of the fruit consumption was done directly with its mouth, without the use of its hands. The kinkajou spent about 10 min feeding on the fruits of M. egregia, consuming roughly 30 % of the infructescence (Figure 2). During this feeding event, few berries were accidentally dropped to the ground, but most were completely consumed by the kinkajou.
After the kinkajou left the site, we explored the area and noticed 2 others ripe infructescences near the one it had consumed, in another individual aroid, although these were located farther from the center of the host tree and show no evidence of frugivory. The next morning, we collected the infructescence and a leaf from the plant to determine its taxonomic identity in the laboratory. Unfortunately, the voucher material was lost before it could be deposited in an academic herbarium. During the collection process, we observed several individuals of M. egregia in the surrounding area, some of which had ripe infructescences.
To our knowledge, this is the first documented case of an ecological interaction between P. flavus and M. egregia, as well as the fourth recorded instance of an Araceae species being consumed by this arboreal and frugivorous mammal. The first report of Araceae fruit consumption by kinkajous was by Bisbal (1986), who noted the consumption of an Araceae infructescence by P. flavus in Venezuela, though the specific species was not identified. Later, in Panamá, Kays (1999) reported a kinkajou feeding on the inflorescence of a Philodendron species. More recently, Cedeño-Fonseca et al. (2020) documented a new interaction between Philodendron and P. flavus, specifically with Philodendron crassispathum, whose infructescence was consumed.
Previous records of P. flavus consuming Araceae plants suggest that this behavior is not random and that the infructescences of Araceae plants could be a potential food resource for arboreal frugivorous mammals. This premise is also supported by the record of Saguinus geoffroyi consuming the infructescence of Monstera adansonii. But how do frugivorous mammals avoid the raphides found in these plants? Cedeño-Fonseca et al. (2020) suggest that in ripe fruits of Monstera species, the styles, which are rich in raphides, detach in large segments or all at once, exposing the pulp surrounding the seed. At this stage, the fruits can be eaten without the ingestion of raphides. The observations of P. flavus and S. geoffroyi support this idea, as in both cases, the fruits were consumed when mature. However, when checked for consumed seeds, some raphides were still present in the tegument of the berries.
In addition to the maturity of the infructescence, our observation indicates that, among the 3 infructescences available in the surrounding area, the one consumed was also the most accessible. It is known that for P. flavus, the location and ease of access to fruit sources often determine fruit selection (Julien-Laferrière 1999), even more so than the degree of fruit maturity (Julien-Laferrière 1993; Kays 1999). In the case of Monstera, we know that only ripe fruits are likely to be consumed by frugivorous mammals (Cedeño-Fonseca et al. 2020). However, in our observation, the ease of access to the plant may have played an important factor, as the other 2 infructescences, which appeared to be mature, showed no signs of frugivory, likely due to their inaccessibility to the kinkajou.
Potos flavus is generally considered a high-canopy frugivore, typically consuming fruits located at around 10-30 m above the ground (Janson and Emmons 1990). In Los Tuxtlas area this species is uncommon at lower heights (Estrada and Coates-Estrada 1985). However, our observation took place at a low height (approximately 6 m). This could be explained by the fact that the tree, where the Kinkajou was observed, was part of a secondary forest, with a low density of tall trees but a high density of M. egregia plants (ca. 150 reproductive individuals/ha in 2024). This abundance of fruit resources may have led the kinkajou to forage at a lower altitude. A subsequent observation, on May 2024 by most of the same team, recorded a kinkajou feeding on Ficus insipida (Moraceae) in this same location, suggesting this is the feeding area of at least one individual.
Apart from the observations on fruit consumption noted above, no specific data on Monstera seed dispersal has been documented. In fact, within the Araceae family, seed dispersal and seed predation remain under-researched (see Vieira and Izar 1999; Cockle 2001; Kobayashi et al. 2017 and with references to Arisaema, Anthurium, Monstera, Rhodospatha, and Philodendron spp.). Most reports on seed dispersal are anecdotal, involving bats, arboreal mammals, and especially birds as the primary dispersers within the family (Madison 1979; Gentry and Dobson 1987; Charles-Dominique and Cockle 2001; Linder and Morawetz 2006; Galindo-González et al. 2008; Suzuki and Maeda 2014; Low 2024). Notably, there is even a report of bears dispersing aroid seeds (Tanaka 2004). Mice and wasps have also been recorded as seed predators in Araceae (Gibernau et al. 2002; Suzuki and Maeda 2014).
It is widely reported that seeds consumed by kinkajous are not negatively affected by digestion (Charles-Dominique et al. 1981; Julien-Laferrière 1993, 1999, 2001; Kays 1999). In some cases, digestion may even enhance germination (Julien-Laferrière 2001). During our observation, the kinkajou ate the entire berries, so it is possible that the seeds of Monstera passed through the Kinkajou’s digestive tract and were later excreted. Reported transit times for food through a kinkajou’s digestive system range from 45 min to 3 hr, with an average of 35 min (Julien-Laferrière 1993). Considering that our observation lasted around 10 min, and the home range estimated for the species in Los Tuxtlas is 8 ha (Estrada and Coates-Estrada 1985), it is possible that the kinkajou traveled a sufficient distance before excreting the seeds of M. egregia. Some partially eaten seeds were found in mildly ingested berries, suggesting that not all consumed seeds survive digestion.
In conclusion, while ecological interactions between P. flavus and Araceae plants are rarely documented, they appear to be more than incidental events. Though our findings are based on a single observation, the study conditions (e. g., high Monstera density and secondary forest characteristics) suggest that this interaction may be relatively common in Los Tuxtlas, Veracruz, México. This highlights the need for further research focused on the frugivory of kinkajous in disturbed habitats, particularly important given that P. flavus is considered a threatened species in México (Diario Oficial de la Federación 2019).
Literature cited
Acebey, A., and T. Krömer. 2008. Diversidad y distribución de Araceae de la Reserva de la Biosfera Los Tuxtlas, Veracruz, México. Revista Mexicana de Biodiversidad 79:465-471.
Bisbal, F. J. 1986. Food habits of some neotropical carnivores in Venezuela (Mammalia, Carnivora). Mammalia 50:329-339.
Cedeño-Fonseca, M., et al. 2020. Notes on frugivory in Monstera and Philodendron (Araceae) from Costa Rica and Panama. Aroideana 43:212-224.
Charles-Dominique, P., et al. 1981. Les mammiferes frugivores arboricoles nocturnes d’une foret guyanaise: inter-relations plantes-animaux. Revue d’ Ecologie 35:341-436.
Charles-Dominique, P., and A. Cockle. 2001. Frugivory and seed dispersal by bats. Pp. 207-216 in Nouragues. Monographiae Biologicae (Bongers F., P. Charles-Dominique, P. M. Forget, and M. Théry, eds.). Springer. Dordrecht, Netherlands.
Cockle, A. 2001. The Dispersal and Recruitment of Cyclanthaceae and Philodendron (Araceae) Understorey Root-Climbing Vines. Pp. 251-264 in Nouragues. Monographiae Biologicae (Bongers F., P. Charles-Dominique, P. M. Forget, and M. Théry, eds.). Springer. Dordrecht, Netherlands.
Coté, G.C., and M. Gibernau. 2012. Distribution of calcium oxalate crystals in floral organs of Araceae in relation to pollination strategy. American Journal of Botany 99:1231-1242.
Croat, T. B., M. Cedeño-Fonseca, and O. O. Ortiz. 2024. Revision of Monstera (Araceae: Monsteroideae) of Central America. Phytotaxa 656:1-197.
Diario Oficial de la Federación. 2019. MODIFICACIÓN del Anexo Normativo III, Lista de especies en riesgo de la Norma Oficial Mexicana NOM-٠٥٩-SEMARNAT-٢٠١٠, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. México. 30 de diciembre de 2010. Available at: https://wwwdofgobmx/nota_detallephp?codigo=5578808andfecha=14/11/2019#gsctab=0
Estrada, A., and R. Coates‐Estrada. 1985. A preliminary study of resource overlap between howling monkeys (Alouatta palliata) and other arboreal mammals in the tropical rain forest of Los Tuxtlas, Mexico. American Journal of Primatology 9:27-37.
Ford, L. S., and R. S. Hoffmann. 1988. Potos flavus. Mammalian Species 321:1-9.
Galindo-González, J., S. Guevara, and V. J. Sosa. 2008. Bat- and bird- generated seed rains at isolated trees in pastures in a tropical rainforest. Conservation Biology 14:1693-1703.
Galvis, N. F., et al. 2024. Notes on the ecology, activity patterns and behavior of the kinkajou (Potos flavus). Mammalia 88:292-298.
Gentry, A. H., and C. H. Dodson. 1987. Diversity and biogeography of neotropical vascular epiphytes. Annals of the Missouri Botanical Garden 74:205-233.
Gibernau, M., et al. 2002. Seed predation in Philodendron solimoesense (Araceae) by chalcid wasps (Hymenoptera). International Journal of Plant Sciences163:1017-1023.
Govaerts, R. 2024. The World Checklist of Vascular Plants (WCVP). Royal Botanic Gardens, Kew Checklist dataset. Available at: https://doiorg/1015468/6h8ucr
Govaerts, R., et al. 2021. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Scientific Data 8:1-10.
Guevara, S., J. Laborde, and G. Sánchez-Ríos. 2004. Los Tuxtlas: El paisaje de la sierra. Instituto de Ecología. Xalapa, México.
Janson, C. H., and L. H. Emmons. 1990. Ecological structure of the nonflying mammal community at Cocha Cashu Biological Station, Manu National Park, Peru. Pp. 314-338 in Four neotropical rainforests (Gentry, A. H., ed.). Yale University Press. New Haven, EE. UU.
Jdeed, L., D. Haddad, and S. Tabash. 2024. Variation and distribution of calcium oxalate crystals in species of the family Araceae. Iranian Journal of Botany 30:62-71.
Julien-Laferrière, D. 1993. Radio-tracking observations on ranging and foraging patterns by kinkajous (Potos flavus) in French Guiana. Journal of Tropical Ecology 9:19-32.
Julien-Laferrière, D. 1999. Foraging strategies and food partitioning in the neotropical frugivorous mammals Caluromys philander and Potos flavus. Journal of Zoology 247:71-80.
Julien-Laferrière, D. 2001. Frugivory and seed dispersal by Kinkajous. Pp. 217-226 in Nouragues. Monographiae Biologicae (Bongers, F., P. Charles-Dominique, P. M. Forget, and M. Théry, eds.). Springer. Dordrecht, Netherlands.
Kays, R. W. 1999. Food preferences of Kinkajous (Potos flavus): a frugivorous carnivore. Journal of Mammalogy 80:589-599.
Kobayashi, T., S. Kitamura, and J. Murata. 2017. Differentiation of fruiting phenology and seed dispersal of Arisaema (Araceae) in Japan: the effect of fruiting season on the rates of fruit removal by avian frugivores. The Journal of Japanese Botany 92:199-213.
Lambert, J. E., et al. 2014. Binturong (Arctictis binturong) and kinkajou (Potos flavus) digestive strategy: implications for interpreting frugivory in Carnivora and Primates. PloS One 9:e105415.
Linder, A., and W. Morawetz. 2006. Seed dispersal by frugivorous bats on landslides in a montane rain forest in Southern Ecuador. Chiroptera Neotropical 12:232-237.
Low, S. L. 2024. Avian frugivory and seed dispersal in Amorphophallus paeoniifolius and Alocasia odora. Tropical Ecology 65:321-329.
Madison, M. 1977. A revision of Monstera (Araceae). Contributions from the Gray Herbarium of Harvard University 207:3-100.
Madison, M. 1979. Protection of developing seeds in neotropical Araceae. Aroideana 2:52-61.
Redford, K. H., A. M. Stearman, and J. C. Trager. 1989. The Kinkajou (Potos flavus) as a Myrmecophage. Mammalia 53:132-134.
Schipper, J. 2007. Camera-trap avoidance by Kinkajous Potos flavus: rethinking the “non-invasive” paradigm. Small Carnivore Conservation 36:38-41.
Suzuki, T., and N. Maeda. 2014. Frugivores of poisonous herbaceous plants Arisaema spp. (Araceae) in the Southern Kanto District, Central Japan. Journal of the Yamashina Institute for Ornithology 45:77-91.
Tanaka, H. 2004. Reproductive biology of Lysichiton camtschatcense (Araceae) in Japan. Aroideana 27:167-171.
Vieira, E. M., and P. Izar. 1999. Interactions between aroids and arboreal mammals in the Brazilian Atlantic rainforest. Plant Ecology 145:75-82.
Villa-Ramírez, B. 1944. Dos nuevos mamíferos de Chiapas. Anales del Instituto de Biología de la Universidad Nacional Autónoma de México 15:319-329.
Associated editor: Gloria Tapia.
Submitted: January 15, 2025; Reviewed: February 13, 2025.
Accepted: March 25, 2025; Published online: April 25, 2025.
Appendix list
Appendices are available at: https://zenodo.org/records/15090507
Appendix 1. Taxonomic list of plant species with which kinkajou (Potos flavus) have ecological interactions in terms of diet in its distribution area.
Appendix 2. Other no-plant items consumed by kinkajou (Potos flavus).
Appendix 3. Video of the kinkajou (Potos flavus) manipulating and consuming the infructescence of Monstera egregia, recorded at Los Tuxtlas Biosphere Reserve, Veracruz, México. https://zenodo.org/records/14597888