Neotropical bats are trophically diverse, including species that feed on arthropods, fruits, nectar, leaves, seeds, vertebrates, and blood (Fenton et al. 1992; Simmons and Conway 2003; Kunz et al. 2011). The use of most of these resources has been extensively documented, varying according to species-specific feeding behaviors (Patterson et al. 2003; Simmons and Conway 2003); however, resources such as seeds and leaves have been less studied, with limited evidence supporting their consumption (Kunz and Ingalls 1994; Kunz and Díaz 1995; Nelson et al. 2005; Bobrowiec and Matos 2010; Ruiz-Ramoni et al. 2011; Wagner et al. 2015; Cordero-Schmidt et al. 2016; da Rocha et al. 2016; Villalobos-Chaves et al. 2016; Pellón 2022; Trujillo et al. 2022; Rodrigues et al. 2023). In the case of leaves, their consumption has been a subject of debate, as the feeding behavior of bats does not fully align with traditional definitions of true folivory (Rodrigues et al. 2023; Muñoz-Romo et al. 2025). Bats typically chew leaf fragments to extract and ingest the juices, discarding the fibrous material (Kunz and Ingalls 1994; Kunz and Díaz 1995; Nelson et al. 2005; Bobrowiec and Matos 2010; Ruiz-Ramoni et al. 2011; Cordero-Schmidt et al. 2016; da Rocha et al. 2016; Rodrigues et al. 2023). Nevertheless, as reports of bat folivory have become more frequent, along with anecdotal observations of bats consuming mature leaves in their entirety without discarding any part; it is increasingly recognized that frugivorous bats may occasionally exhibit specific folivorous behaviors (Rodrigues et al. 2023; Muñoz-Romo et al. 2025). To date, folivory has been documented in five species within the genera Artibeus, Platyrrhinus, and Carollia (Rodrigues et al. 2023).
Folivory in Neotropical fruit bats has been documented since 1957 (Greenhall 1957) and continues to be an active area of research, with several key findings that have enhanced our understanding and led to hypotheses regarding leaf consumption (Kunz and Ingalls 1994; Kunz and Díaz 1995; Nelson et al. 2005; Bobrowiec and Matos 2010; Ruiz-Ramoni et al. 2011; Cordero-Schmidt et al. 2016; da Rocha et al. 2016; Rodrigues et al. 2023). Initially, evidence of leaf consumption was gathered through the analysis of discarded material, such as partially consumed leaves and oral pellets, and was further supported by direct observations of bats feeding (Zortéa & Mendes 1993; Kunz & Ingalls 1994; Kunz & Diaz 1995; Nogueira & Peracchi 2003; Ruiz-Ramoni et al. 2011; Cordero-Schmidt et al. 2016; Duque-Márquez et al. 2019). The use of complementary research methodologies, such as camera devices to monitor colonies and feeding roosts, has also made significant contributions to our understanding of this behavior (Silvestre et al. 2016; da Rocha et al. 2016; Pereira et al. 2017; Muñoz-Romo et al. 2025). Experimental tests have also provided additional insights into the folivorous habits of bats (Nelson et al. 2005).
The factors driving folivory in fruit-eating bats remain poorly understood (Rodrigues et al. 2023; Muñoz-Romo et al. 2025). Some studies suggest that nutritional needs, such as carbohydrates, proteins, and minerals lacking in fruits, may drive this behavior (Kunz and Ingalls 1994; Kunz and Díaz 1995; Nelson et al. 2005; Bobrowiec and Matos 2010). Other factors, including water scarcity in dry habitats (Cordero-Schmidt et al. 2016; Rodrigues et al. 2023) and fruit availability tied to plant phenology (da Rocha et al. 2016), have also been proposed. It is important to note that none of these factors influencing folivory in bats is mutually exclusive; rather, folivory can result from a combination of all three. However, much of the existing literature remains descriptive and lacks detail (Ruiz-Ramoni et al. 2011; Duque-Márquez et al. 2019), highlighting a significant knowledge gap that requires further investigation.
This study aims to report, for the first time, leaf consumption by Artibeus lituratus in Guatemala, documenting the diversity of food resources consumed at feeding perches in Parque Nacional Naciones Unidas (PNNU) in central Guatemala, a region where this behavior has not been previously recorded.
Parque Nacional Naciones Unidas (PNNU), located between the municipalities of Villa Nueva and Amatitlán in central Guatemala, spans 372 ha with elevations ranging from 1,190 to 1,330 m. The region receives annual rainfall between 1,100 and 1,349 mm, with temperatures typically ranging from 20 to 23 °C. PNNU is part of ecosystems classified as Premontane Humid Forest Ecosystem, which has predominantly pine-oak species associations (IARNA-URL 2018). The area has been significantly impacted by anthropogenic activities, leading to considerable changes in its forest structure. In most areas, native vegetation has been replaced by plantations intended for forest restoration, featuring non-native species such as cypress (Hesperocyparis lusitanica), eucalyptus (Eucalyptus sp.), casuarina (Casuarina sp.), pine (Pinus sp.), and various fruit trees and schrubs (CONAP 2005).
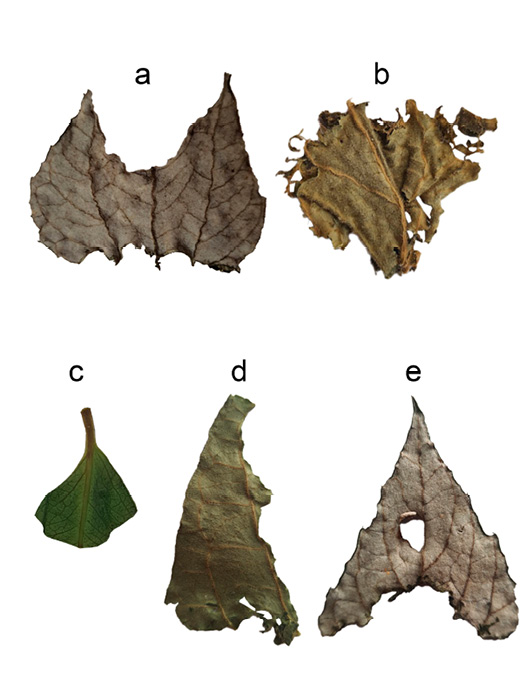
Fruit, seed, and nibbled leaf samples were collected from feeding perches frequented every night by A. lituratus, where evidence such as leaf and fruit pellets, seed and fruit remains, and partially nibbled leaves were found on tables below the perches (Figure 1). Sampling was conducted twice a week, early in the morning, from September to December 2023. Each sample was rinsed with water, disinfected with 70% alcohol, dried, and then placed in a waxed paper envelope for preservation (Morales-Pizarro et al. 2023).
Seed identification was conducted using the ‘Index Seminum’ seed reference collection from the Botanical Garden at Universidad de San Carlos de Guatemala (Portal de Biodiversidad de Guatemala 2025). For leaf identification, diurnal surveys were conducted to collect plants whose leaves were potentially consumed by the bats. Bats were identified through multiple direct observations of individuals perching on the feeding perches, which were consistently used each night. To confirm these observations, bats were also captured using mist nets set up around the feeding perches. Species identification was conducted using regional field guides (Medellín et al. 2008; Reid 2009).
To assess the diversity of fruits and leaves consumed at the feeding perches, we analyzed the collected samples, categorizing the remains of each identified food item. Leaf consumption was classified into five distinct patterns: basal, apical, edge, apical-basal, and vein. In the basal pattern, bites are concentrated at or along the leaf base; in the apical pattern, bites occur at or along the leaf apex. The edge pattern involves bites along the entire leaf margin, while the apical-basal pattern features bites at both the apex and base, sparing the middle and margins. The vein pattern includes bites along the midrib and adjacent areas of the lamina. To quantify the proportion of each leaf consumed, we classified the patterns based on whether less than or greater than 50% of the leaf was consumed.
A total of 24 food items were documented at nocturnal feeding perches within PNNU, consisting of leaves of 9 species and seeds of 15 species (Table 1; Figure 2). October exhibited the highest species richness, with leaves of 7 species and seeds of 11 species, while December had the lowest diversity, with only seeds of 4 species and no leaf consumption.
Among the fruit species observed, Spondias purpurea, Syzygium jambos, Ficus sp., and Solanum sp. were consistently present across all months. Rhaphiolepis bibas was exclusive to September and October. Other seeds, including Areca sp., Casearia sp., Panicum sp., and Morphospecies 1–3, were recorded in only one month. The 9 leaf species observed included those from the genera Sinclairia, Solanum and Lycianthes, along with other species cataloged as Morphospecies (Morphospecies 1–5). Leaf consumption was documented from September to November, with no evidence of leaves being consumed in December. Leaves of Solanum, Sinclairia and Morphospecies 3 were the most frequently consumed (Figure 2).
The number of nibbled leaves by A. lituratus varied across months, with the highest number in October (n = 24), followed by September (n = 11) and November (n = 3). Five distinct leaf-ingestion patterns were identified (Figure 3), with the basal pattern being the most predominant (Table 2). The proportion of the leaf consumed varied among species, with consumption exceeding 50% in 7 of the 9 leaf species. Most of the consumed leaves shared a smooth and pubescent surface, with trichomes arranged in various configurations. Of the 9 leaf species, 7 exhibited trichomes in varying densities on both surfaces (Supplemental material 1).
This study provides novel insights into the feeding ecology of A. lituratus in Guatemala, highlighting its dietary plasticity and ability to exploit resources underutilized by other frugivorous bats. Notably, Sinclairia sublobata leaves were recorded for the first time as a dietary resource for any bat species. Furthermore, the frequent consumption of Solanum leaves, previously documented in the diet of A. lituratus (Duque-Márquez et al. 2019; Rodrigues et al. 2023), extends the knowledge of the consumption of leaf species from this genus into the region.
A distinct pattern of leaf consumption was observed, with a preference for the basal section (Figure 2 and Table 2). This is consistent with previous studies, which suggest that the higher concentrations of nutrients and water, combined with lower fiber content, make the basal portion more attractive to frugivorous bats (Zortéa and Mendes 1993; Kunz and Diaz 1995; Ruiz-Ramoni et al. 2011; Cordero-Schmidt 2016; Duque-Márquez et al. 2019). The uneven distribution of nutrients and water, typically higher at the base, likely drives this preference (Sandars et al. 2010). In the case of the 9 leaf species, 7 exhibited trichomes on both surfaces, with densities varying across species. These structures, commonly developed by some plants to prevent herbivory (São-João and Raga 2016; Karabourniotis et al. 2020), seem to have no effect on A. lituratus.
The evidence of feeding behavior by A. lituratus in the PNNU showed consistent fruit consumption over the four months of observation, whereas leaf consumption was observed in only three of those months. Notably, the evidence of leaf consumption indicated a gradual decline over time; however, the available observations are insufficient to establish this as a pattern. Among the fruits, species from the families Solanaceae (Solanum sp.), Moraceae (Ficus sp.), and Anacardiaceae (Spondias purpurea) were the most frequently consumed, while for leaves, the families Solanaceae (Solanum sp.) and Asteraceae (Liabum sp.) were the most common.
Artibeus lituratus is exploiting plant species that were once part of the restoration programs in the PNNU, including exotic plants such as Areca sp. (Arecaceae), R. bibas (Rosaceae), and S. jambos (Myrtaceae), which were intentionally introduced to support the park’s biodiversity (CONAP 2005). The recovery zone, primarily resulting from human-mediated ecological restoration, now provides these resources. The findings from this study offer evidence of the integration of biodiversity within these restoration efforts and highlight the species’ flexibility in utilizing both native and non-native resources.
Acknowledgements
We are grateful to the PNNU administration, particularly to Director C. Mejía, for opening the doors and allowing us to obtain the samples during the study period; to the park rangers A. Godoy, F. Godoy, S. Morales and D. García for storing the samples and accompanying us in the field; to the biologist J. Jiménez and the Index seminum of the USAC Jardín Botánico for helping us with the identification of the specimens. We are grateful to the Fundación Defensores de la Naturaleza (FDN) for making this study possible.
Literature cited
Bobrowiec, P. E., and R. Matos. 2010. Leaf-consuming behavior in the big fruit-eating bat, Artibeus lituratus (Olfers, 1818) (Chiroptera: Phyllostomidae), in an urban area of Southeastern Brazil. Chiroptera Neotropical 16:595-599.
Consejo Nacional De Áreas Protegidas (Conap). 2005. Plan Maestro 2006-2010: Parque Nacional Naciones Unidas. https://conap.gob.gt/wp-content/uploads/2019/10/PM-PN-Naciones-Unidas.pdf. Accessed on April 23, 2024.
Cordero-Schmidt, E., et al. 2016. Are leaves a good option in Caatinga’s menu? First record of folivory in Artibeus planirostris (Phyllostomidae) in the semiarid forest, Brazil. Acta Chiropterologica 18:489-497.
dA Rocha, P. A., et al 2016. Consumption of leaves by Platyrrhinus lineatus (Chiroptera, Stenodermatinae): are these bats primarily frugivorous or broadly phytophagous? Zoology 121:44-48.
Duque-Márquez, A., et al 2019. Bat folivory in numbers: how many, how much, and how long? Acta Chiropterologica 21:183-191.
Fenton, M. B., et al. 1992. Phyllostomid bats (Chiroptera: Phyllostomidae) as indicators of habitat disruption in the Neotropics. Biotropica, 440-446.
Greenhall, A. M. 1957. Food preferences of Trinidad fruit bats. Journal of Mammalogy 38(3):409–410.
IARNA-URL (Instituto de Investigación y Proyección sobre Ambiente Natural y Sociedad de la Universidad Rafael Landívar). 2018. Ecosistemas de Guatemala basado en el sistema de clasificación de zonas de vida. IARNA-URL, Guatemala City, Guatemala. 122 pp.
Karabourniotis, G., et al. 2020. Protective and defensive roles of non-glandular trichomes against multiple stresses: structure–function coordination. Journal of Forestry Research 31(1): 1-12.
Kunz, T. H., and K. A. Ingalls. 1994. Folivory in bats: an adaptation derived from frugivory. Functional Ecology 8:665-668.
Kunz, T. H., and C. A. Díaz. 1995. Folivory in Fruit-eating bats, with new evidence from Artibeus jamaicensis (Chiroptera: Phyllostomidae). Biotropica 27:106-120.
Kunz, T. H., et al. 2011. Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223:1–38.
Medellín, R., et al. 2008. Identificación de los murciélagos de México: Clave de campo. Instituto de Ecología, UNAM, Ciudad de México, México. 78 pp.
Morales Pizarro, A., et al. 2023. Efecto de diferentes dosis de ácido giberélico en la germinación de papaya (Carica papaya L.) variedad criolla. Chilean Journal of Agricultural & Animal Sciences 39:392–400.
Muñoz-Romo, M., et al. 2025. Actual folivory in bats: whole leaves as a formerly dismissed trophic item and a potential avenue for self-medication. Behaviour 1:1–18.
Nelson, S. L., et al. 2005. Folivory in fruit bats: leaves provide a natural source of calcium. Journal of Chemical Ecology 31:1683-1691.
Nogueira, M. R., and A. L. Peracchi. 2003. Fig-seed predation by 2 species of Chiroderma: discovery of a new feeding strategy in bats. Journal of Mammalogy 84:225–233
Nogueira, M. R., et al. 2005. Leaf-vein traits in bats - an adaptation against herbivory? Acta Chiropterologica 7:89-105.
Nogueira, M. R., et al. 2005. Ecomorphological analysis of the masticatory apparatus in the seed-eating bats, genus Chiroderma (Chiroptera: Phyllostomidae). Journal of Zoology 266:355–364.
Patterson, B. D., et al. 2003. Trophic strategies, niche partitioning, and patterns of ecological organization. In: T. H. Kunz and M. B. Fenton (Eds.). Bat ecology. University of Chicago Press, Chicago: 536–579.
Pellón, J. 2022. Fruits consumed by phyllostomid bats in a Peruvian Yungas Forest: new dietary items for Chiroderma salvini and Lonchophylla handleyi. Mammalia 86:261–265.
Pereira, A. S., et al. 2017. Consumption of leaves by Carollia perspicillata (Chiroptera, Phyllostomidae): a new dimension of the species’ feeding ecology. Mammalia, 82(1): 88–91.
Portal de Biodiversidad de Guatemala. 2025. Colección de Frutos y Semillas “Index Seminum”, Jardín Botánico-CECON-USAC. Occurrence dataset (ID: 97c4811e-91ea-41ab-a9b8-396e925b3a25) accessed via the Biodiversidad de Guatemala Portal, /portal, 2025-03-14).
Reid, F. A. 2009. A field guide to the mammals of Southeast Mexico and Central America. 2nd ed. Oxford University Press, New York, USA. 346 pp.
Rodrigues, T. M., et al. 2023. A review of folivory by Neotropical bats. Interfaces Científicas - Saúde e Ambiente 9:29-44.
Ruiz-Ramoni, D., et al. 2011. Folivory in the giant fruit-eating bat Artibeus amplus (Phyllostomidae): a non-seasonal phenomenon. Acta Chiropterologica 13:195-199.
Sandars, J., et al. 2010. Changes in water content and distribution in Quercus ilex leaves during progressive drought assessed by in vivo 1H magnetic resonance imaging. BMC Plant Biology 10:1-12.
São-João, R. E., and Raga M. 2016. Mecanismo de defensa das plantas contra o ataque de insetos sugadores (Documento Técnico 23). Governo do Estado de São Paulo. http://www.biologico.sp.gov.br/uploads/docs/dt/insetos_sugadores.pdf. Accessed on April 1, 2025.
Silvestre, S. M., et al. 2016. Diet and seed dispersal potential of the white-lined bat, Platyrrhinus lineatus (E. Geoffroy, 1810), at a site in northeastern Brazil. Studies on Neotropical Fauna and Environment 51: 37-44.
Simmons, N. B., & Conway, T. M. 2003. Evolution of ecological diversity in bats. In: T. H. Kunz and M. B. Fenton (Eds.). Bat ecology. University of Chicago Press, Chicago: 493–535.
Trujillo, L. A., et al. 2022. Notes on the life history of Centurio senex (Chiroptera: Phyllostomidae) from northern Central America. Mammalia 86:468–473.
Villalobos-Chaves, D., et al. 2016. Seed predation by the wrinkle-faced bat Centurio senex: a new case of this unusual feeding strategy in Chiroptera. Journal of Mammalogy 97:726–733.
Wagner, I., et al. 2015. Cheating on the mutualistic contract: nutritional gain through seed predation in the frugivorous bat Chiroderma villosum (Phyllostomidae). Journal of Experimental Biology 218:1016–1021.
Zortéa, M., and S. L. Mendes. 1993. Folivory in the Big fruit-eating Bat, Artibeus lituratus (Chiroptera: Phyllostomidae) in eastern Brazil. Journal of Tropical Ecology 9:117-120.
Associated editor: Daily Martínez Borrego.
Submitted: December 18, 2024; Reviewed: May 02, 2025.
Accepted: May 04, 2025; Published on line: June 16, 2025.