Sound communication among conspecific mammals is quite common and facilitates various behavioral interactions. The Bolivian bamboo rat, Dactylomys boliviensis, like many other mammals, uses this system to mark its territory and attract females. We occasionally recorded the complete song of this species within Carrasco National Park, an area close to the type locality, representing the first record of the species for the park. We compared our record with other calls from further northwest, near the border with Peru. The call consists of a strong staccato, is divided into 2 parts, and the dominant frequency is 1.16 kHz. Our results reveal considerable geographic variation across the species’ range. The variation found may be influenced by factors such as individual size, soundscape limitations, changes in song structure due to distance, or even species differentiation. This record presents an opportunity for the scientific community to further investigate the causes of this call variation.
Key words: Bioacustics; call structure; communication; intraspecific variation; type locality.
La comunicación entre los conespecíficos mamíferos a través del sonido es bastante común, y facilita diferentes interacciones comportamentales entre ellos. La rata de bambú boliviana, Dactylomys boliviensis, utiliza este sistema de comunicación para delimitar su territorio y atraer a las hembras. De manera ocasional grabamos el canto completo de esta especie dentro el Parque Nacional Carrasco, cerca de la localidad tipo, constituyéndose en el primer registro de la especie para el parque. Hacemos comparaciones con otros cantos registrados mucho más al noroeste cerca la frontera con Perú, encontrando diferencias significativas en tiempos y estructura. El canto consiste en un staccato fuerte, está dividido en 2 partes, y la frecuencia dominante es 1.16 kHz. Nuestros resultados muestran una gran variación geográfica del canto entre los individuos. La variación encontrada puede estar ocasionada por diferentes razones como el tamaño de los individuos, restricciones en el paisaje sonoro, cambios en la estructura del canto debido a la distancia, o incluso una diferenciación específica. Esto abre una oportunidad a la comunidad científica para investigar con mayor profundidad las causas de esta variación en el canto.
Palabras clave: Bioacústica; comunicación; estructura del canto; localidad tipo; variación intraespecífica.
Sound is a key communication system used by mammals, playing an important role in coordinating behavioral interactions between conspecifics (Feldhamer et al. 2007). It is employed to transmit different messages, such as defending territory or attracting the opposite sex. These calls can range from simple sound pulses (staccato) to more complex multi-note songs. In some species, these songs may even be synchronized between males and females, resembling duets (Fitch 2006; Vanderhoff and Bernal-Hoverud 2022).
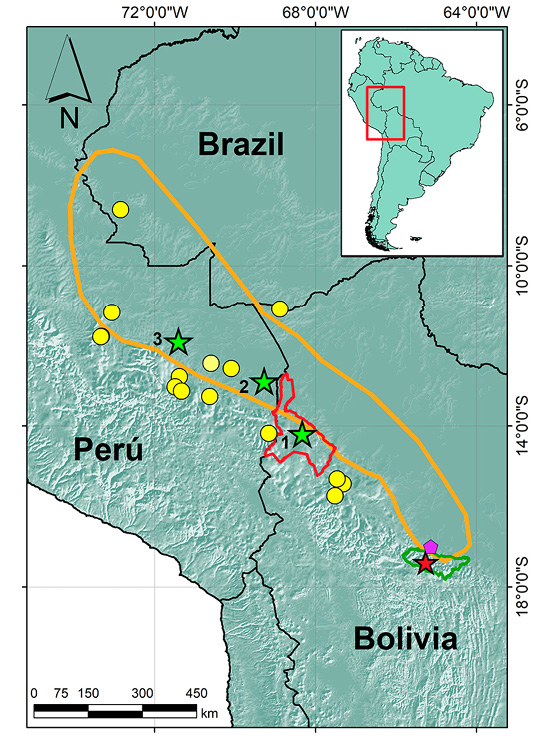
The Bolivian bamboo rat Dactylomys boliviensis Anthony, 1920, is a mammal known for its loud territorial calls, which can be heard from hundreds of meters away in the forest (Dunnum and Salazar-Bravo 2004). Classified as ‘Least Concern’ by the International Union for Conservation of Nature IUCN (Vivar 2016), it is distributed between 200 and 2,132 m on the eastern slopes of the central Andes, in the countries of Bolivia, Perú, and western Brazil (Dunnum and Salazar-Bravo 2004; Abreu-Júnior et al. 2016; Vivar 2016; Figure 1). Vanderhoff and Bernal-Hoverud (2022) analyzed the vocalization of this species for the first time, using a recording from Madidi National Park and Integrated Management Natural Area (hereafter Madidi NP) in north-western Bolivia. Their analysis focused on the synchronized duet calling of the female and male, providing a general description. However, they did not conduct an in-depth analysis of the dominant frequencies, pulse timing, or interpulse intervals of the call.
During a herpetological assessment in Carrasco National Park and Integrated Management Natural Area (hereafter Carrasco NP), we occasionally recorded the calls of D. boliviensis. This is the first documented occurrence of this arboreal mammal in Carrasco NP (Rumiz et al. 1998). Furthermore, we provide the first detailed description of its calls near the type locality at Misión San Antonio, Río Chimoré (Dunnum and Salazar-Bravo 2004), which is 45 km from our recording site (Figure 1).
Carrasco NP is located in the Yungas Ecoregion, which is characterized by a humid evergreen forest, with average annual temperatures between 7-24 °C and an approximate annual precipitation between ١,٥٠٠ to > 6,000 mm, thus considered the wettest area in Bolivia (Ibisch et al. 2003). It presents a high diversity of plant families such as Araliaceae, Bromeliaceae, Euphorbiaceae, Lauraceae, Melastomataceae, Myrtaceae, Orchidaceae, Piperaceae, Podocarpaceae, Rubiaceae, among others, and typical tree genera in the region are Acalypha, Alchornea, Aniba, Cinchona, Cyathea, Ficus, Inga, Nectandra, Persea, Solanum, and Trichilia (Ibisch et al. 2003). Figure 2 shows the characteristics of the area where the recordings of D. boliviensis were made, featuring typical vegetation of the Yungas ecoregion.
Calls were recorded with a SONY ICD-PX820 digital recorder, temperature and humidity were recorded with an HTC-2 digital thermohygrometer. We based our song description on the terminology used by Emmons (1981) and Angulo (2006), and the song was processed with Adobe Audition 2022. The call parameters were compared using a Kruskal-Wallis test followed by Dunn’s post-hoc test with Bonferroni adjustment, using RStudio (RStudio Team 2022). Audio recordings have been uploaded to iNaturalist (https://www.inaturalist.org), and wav files are available at https://doi.org/10.5281/zenodo.15277249.
To examine potential geographic variation and understand how these calls differ across the species’ range, we compared our recordings of D. boliviensis with the one reported by Vanderhoff and Bernal-Hoverud (2022) from Madidi NP, as well as with recordings from Laguna Chica and Cocha Cashu in Peru, deposited in the Macaulay Library at the Cornell Lab (https://www.macaulaylibrary.org).
On July 30, 2024 at 18:59 hr, we recorded an individual of D. boliviensis from an estimated distance of 60 m, at the coordinates 17° 23’ 17.72’’ S, 65° 15’ 37.49’’ W, and at an elevation of 1,099 m. The temperature was 18.3 °C, and the humidity ٨٢ %. This recording did not have enough quality to measure the duration, period, and amplitude of each pulse; however, it was useful to observe the call structure. The recording file is deposited at https://www.inaturalist.org/observations/247818581 (wav file available at https://doi.org/10.5281/zenodo.15277249).
On July 31, 2024, at 19:29 hr, we recorded an individual of D. boliviensis at the coordinates 17° 23’ 11.53’’ S, 65° 15’ 36.3’’ W, and at an elevation of 1,055 m. This recording was of high quality and was obtained from a distance of 6 m. The temperature was 21.2 °C, and the humidity ٧٩ %. The call was recorded in a typical Dactylomys habitat, a bamboo thicket of what we assume is the genus Guadua (Vanderhoff and Bernal-Hoverud 2022). The intervals between songs were long, approximately 1 hr, similar to those reported by Vanderhoff and Bernal-Hoverud (2022) in Madidi NP, and in some cases we could hear a reply from a second individual, with a separation of more than 190 m between them. Recording file is deposited at https://www.inaturalist.org/observations/247818583 (wav file available at https://doi.org/10.5281/zenodo.15277249).
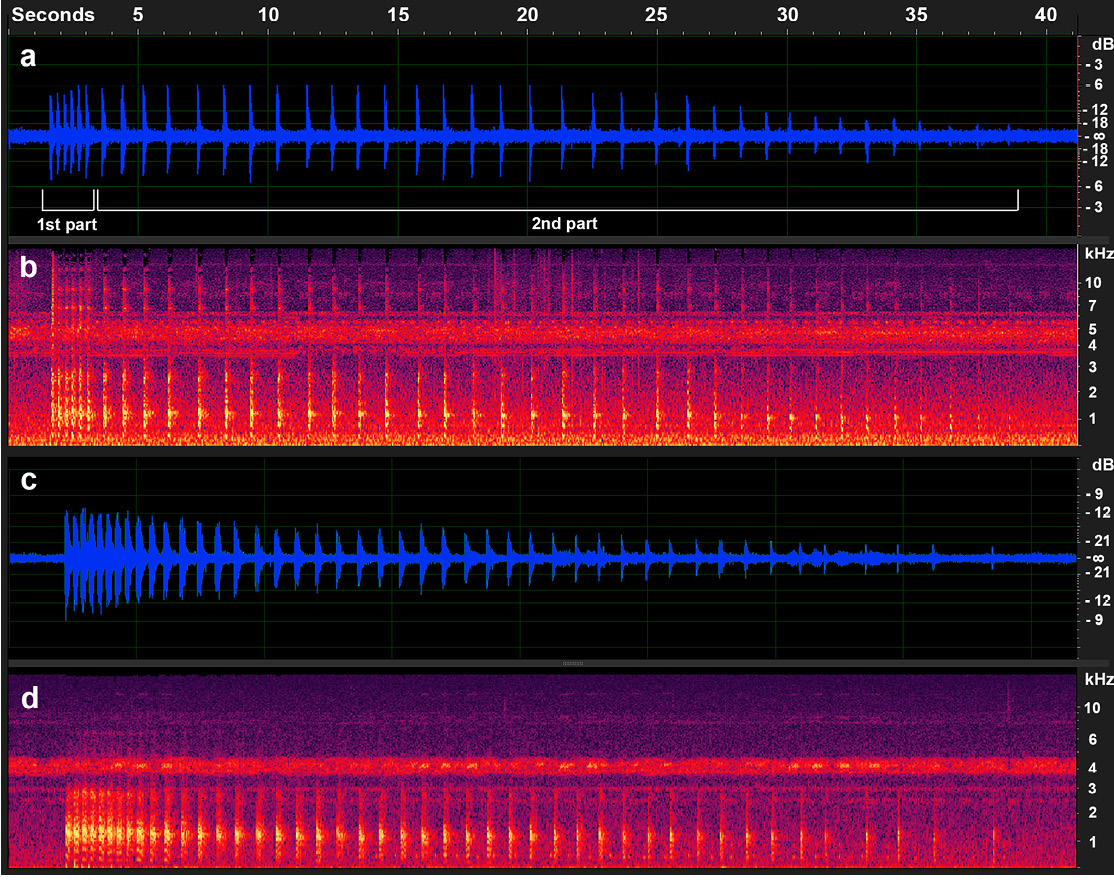
The call consists of a strong staccato with 40 pulses lasting 36.99 s and is divided in 2 parts (Figure 3a). The first part is an initial call lasting 1.9 s, made up of 6 consecutive pulses. The second part lasts 34.99 s and contains 34 pulses, which are spaced much farther apart than in the first part (Figure 3a). The dominant frequency is 1.16 kHz (Figure 3b). In contrast to other recordings from farther north (Madidi NP, Laguna Chica, Cocha Cashu), these are a single continuous call with no distinguishable parts (call from Madidi NP as an example, Figure 3c and 3d). These northern recordings feature a strong staccato of 32 to 45 pulses, with a duration ranging from 8.22 to 36.50 s and a dominant frequency between 0.99 and 1.21 kHz.
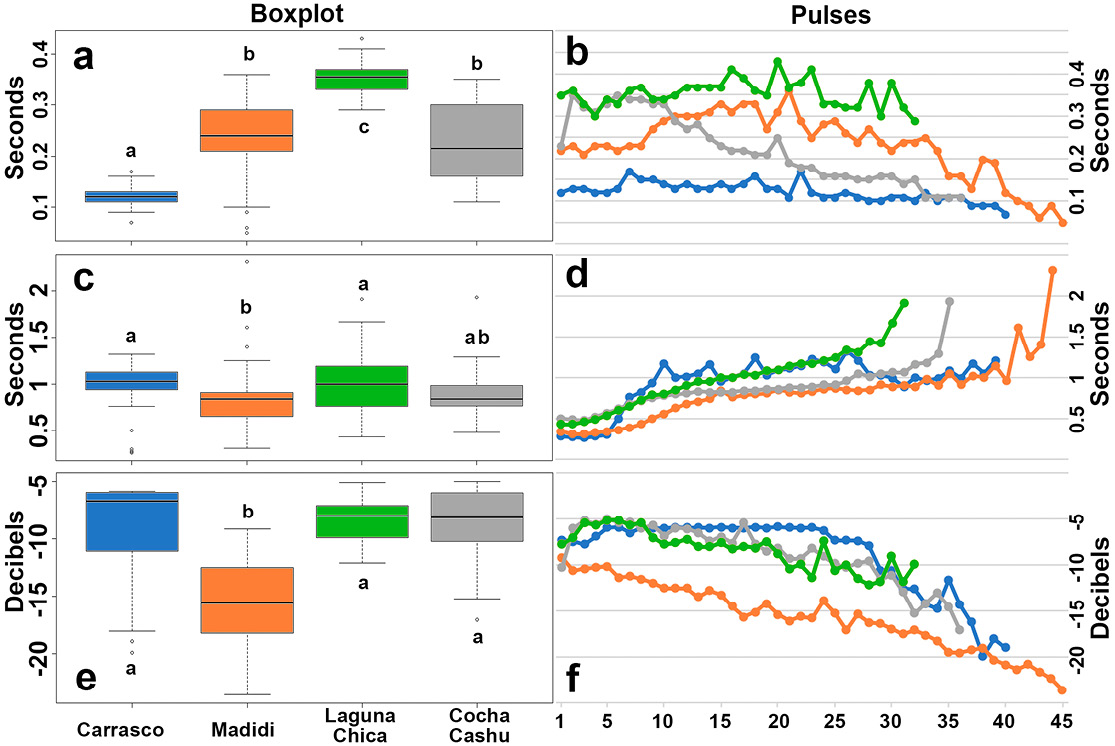
There are significant differences in pulse duration between calls (X = 96.87; d.f. = 3; P < 0.001). The pulses in the call from Carrasco NP are the shortest, averaging 0.12 ± 0.02 s, compared to those from Laguna Chica, which average 0.35 ± 0.03 s. Both show significant differences compared to the other calls (Table 1 and Figure 4a). In all cases, the pulse duration follows a pattern of slight increase in the middle of the call, followed by a decrease toward the end (Figure 4b).
Although the intervals between pulses appear similar, there are significant differences (X = 13.61; d.f. = 3; P < 0.01). The pulse intervals from Madidi NP differ significantly from those at Carrasco NP and Laguna Chica, while the intervals at Cocha Cashu show no significant differences compared to the others (Figure 4c). With the exception of Carrasco NP, all sites exhibit increasing intervals toward the end of the call compared to the beginning (Figure 4d).
In terms of amplitude, the call from Madidi NP differs significantly from the others (X = 65.78; d.f. = 3; P < 0.001; Figure 4e). The call from Carrasco NP maintains a nearly consistent intensity up until pulse 23, after which it begins to decrease. In contrast, the song from Madidi NP shows a gradual decline in intensity from the very beginning (Figure 4f).
We provide a detailed description of the call of D. boliviensis, which is the first record of this species in Carrasco NP (Rumiz et al. 1998). Notably, this call was recorded very close to the species’ type locality, making it an important reference for future studies on the natural history and acoustic behavior of D. boliviensis. We also compared it to calls recorded from the same species in Madidi NP (Bolivia), Laguna Chica (Peru), and Cocha Cashu (Peru), where we found significant differences in both timing and structure. These variations confirm that the call of D. boliviensis is highly variable, as previously noted by Emmons (1981). This variability may be influenced by several factors, including differences in individual size, variations in the soundscape where individuals must adapt to available frequencies, changes in song structure due to distance, or even potential species-specific differentiation (Pijanowski et al. 2011; Gustison and Townsend 2015).
Given the limited availability of complete song recordings for the genus Dactylomys, we are far from fully understanding the causes of this variation. However, the proximity of this record to the type locality adds crucial value to our knowledge of the species’ vocal repertoire, underscoring the need for further attention from the scientific community.
Acknowledgements
The authors wish to express their gratitude to the protected area Carrasco NP (Parque Nacional y Área Natural de Manejo Integrado Carrasco), Empresa Nacional de Electricidad Bolivia (ENDE) Transmisión, and ENDE Valle Hermoso for their commitment to environmental conservation.
Literature cited
Abreu-Júnior, E., D., et al. 2016. Marsupials and rodents (Didelphimorphia and Rodentia) of upper Rio Acre, with new data on Oxymycterus inca Thomas, 1900 from Brazil. Check List 12:1-16.
Angulo, A. 2006. Fundamentos de bioacústica y aspectos prácticos de grabaciones y análisis de cantos. Pp. 93-134 in Técnicas de inventario y monitoreo para los anfibios de la región tropical Andina. (Angulo, A., et al., eds.). Conservación Internacional. Bogotá, Colombia.
Dunnum, J. L., and J. Salazar-Bravo. 2004. Dactylomys boliviensis. Mammalian Species 745:1-4.
Emmons, L. H. 1981. Morphological, ecological, and behavioral adaptations for arboreal browsing in Dactylomys dactylinus (Rodentia, Echimyidae). Journal of Mammalogy 62:183-189.
Feldhamer, G. A., et al. 2007. Mammalogy: adaptation, diversity, ecology. Third edition. The Johns Hopkins University Press. Baltimore, U.S.A.
Fitch, W. T. 2006. Production of vocalizations in mammals. Visual Communication 3:115-121.
GBIF. 2024. Dactylomys dactylinus. In: GBIF.org. Occurrence Download https://doi.org/10.15468/dl.93rypu. Accessed on August 22, 2024.
Gustison, M. L., and S. W. Townsend. 2015. A survey of the context and structure of high- and low-amplitude calls in mammals. Animal Behaviour 105:281-288.
Ibisch, P. L., et al. 2003. Ecoregiones y ecosistemas. Pp. 47-88 in Biodiversidad: La riqueza de Bolivia. Estado de conocimiento y conservación (Ibisch, P. L., and G. Mérida, eds.). Ministerio de Desarrollo Sostenible. Editorial FAN. Santa Cruz de la Sierra, Bolivia.
Pijanowski, B. C., et al. 2011. Soundscape Ecology: The Science of Sound in the Landscape. BioScience 61:203-216.
RStudio Team 2022. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/.
Rumiz, D. I., C. F. Eulert, and R. Arispe. 1998. Evaluación de la diversidad de mamíferos medianos y grandes en el Parque Nacional Carrasco (Cochabamba - Bolivia). Revista Boliviana de Ecología 4:77-90.
Vanderhoff, E. N., and N. Bernal-Hoverud. 2022. Perspectives on antiphonal calling, duetting and counter-singing in non-primate mammals: An overview with notes on the coordinated vocalizations of bamboo rats (Dactylomys spp., Rodentia: Echimyidae). Frontiers in Ecology and Evolution 10:906546.
Vivar, E. 2016. Dactylomys boliviensis. In: UICN 2024. The IUCN Red List of Threatened Species. Version 2024.1. www.iucnredlist.org. Accessed on September 15, 2024.
Associated editor: Daily Martínez Borrego.
Submitted: October 23, 2024; Reviewed: May 05, 2025.
Accepted: May 06, 2025; Published on line: June 25, 2025.